| Pharmaceutical Information |
| Drug Name |
Ketamine |
| Drug ID |
BADD_D01228 |
| Description |
Ketamine is an NMDA receptor antagonist with a potent anesthetic effect.[A31869] It was developed in 1963 as a replacement for phencyclidine (PCP) by Calvin Stevens at Parke Davis Laboratories. It started being used for veterinary purposes in Belgium and in 1964 was proven that compared to PCP, it produced minor hallucinogenic effects and shorter psychotomimetic effects. It was FDA approved in 1970, and from there, it has been used as an anesthetic for children or patients undergoing minor surgeries but mainly for veterinary purposes.[L1332] |
| Indications and Usage |
Ketamine is indicated as an anesthetic agent for recommended diagnostic and surgical procedures. If skeletal muscle relaxation is needed, it should be combined with a muscle relaxant. If the surgical procedure involves visceral pain, it should be supplemented with an agent that obtunds visceral pain. Ketamine can be used for induction of anesthesia prior other general anesthetic agents and as a supplement of low potency agents.[L1336, FDA label]
Reports have indicated a potential use of ketamine as a therapeutic tool for the management of depression when administered in lower doses.[A31873] These reports have increased the interest for ketamine in this area and several clinical trials are launched for this indication.[L1337, A31874] |
| Marketing Status |
approved; vet_approved |
| ATC Code |
N01AX03 |
| DrugBank ID |
DB01221
|
| KEGG ID |
D08098
|
| MeSH ID |
D007649
|
| PubChem ID |
3821
|
| TTD Drug ID |
D0UM7O
|
| NDC Product Code |
Not Available |
| UNII |
690G0D6V8H
|
| Synonyms |
Ketamine | 2-(2-Chlorophenyl)-2-(methylamino)cyclohexanone | CI-581 | CI 581 | CI581 | Ketalar | Ketaset | Ketanest | Calipsol | Kalipsol | Calypsol | Ketamine Hydrochloride |
|
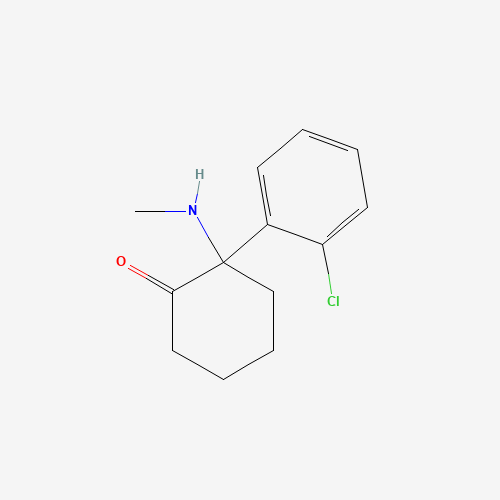
| Chemical Information |
| Molecular Formula |
C13H16ClNO |
| CAS Registry Number |
6740-88-1 |
| SMILES |
CNC1(CCCCC1=O)C2=CC=CC=C2Cl |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

