| Pharmaceutical Information |
| Drug Name |
Enalapril |
| Drug ID |
BADD_D02425 |
| Description |
Enalapril is a prodrug belonging to the angiotensin-converting enzyme (ACE) inhibitor drug class that works on the renin-angiotensin-aldosterone system, which is responsible for the regulation of blood pressure and fluid and electrolyte homeostasis. Enalapril is an orally-active and long-acting nonsulphydryl antihypertensive agent that suppresses the renin-angiotensin-aldosterone system to lower blood pressure. It was developed from a targeted research programmed using molecular modelling.[A18459] Being a prodrug, enalapril is rapidly biotransformed into its active metabolite, [enalaprilat], which is responsible for the pharmacological actions of enalapril. The active metabolite of enalapril competitively inhibits the ACE to hinder the production of angiotensin II, a key component of the renin-angiotensin-aldosterone system that promotes vasoconstriction and renal reabsorption of sodium ions in the kidneys. Ultimately, enalaprilat works to reduce blood pressure and blood fluid volume.
Commonly marketed under the trade name Vasotec, enalapril was first approved by the FDA in 1985 for the management of hypertension, heart failure, and asymptomatic left ventricular dysfunction. It is also found in a combination product containing [hydrochlorothiazide] that is used for the management of hypertension. The active metabolite enalaprilat is also available in oral tablets and intravenous formulations for injection. |
| Indications and Usage |
For the treatment of essential or renovascular hypertension and symptomatic congestive heart failure. It may be used alone or in combination with thiazide diuretics. |
| Marketing Status |
Not Available |
| ATC Code |
C09AA02 |
| DrugBank ID |
DB00584
|
| KEGG ID |
D07892
|
| MeSH ID |
D004656
|
| PubChem ID |
5388962
|
| TTD Drug ID |
D00SEB
|
| NDC Product Code |
69238-2141 |
| Synonyms |
Enalapril | MK-421 | MK 421 | MK421 | Renitec | Renitek | Enalapril Maleate | Maleate, Enalapril |
|
| Chemical Information |
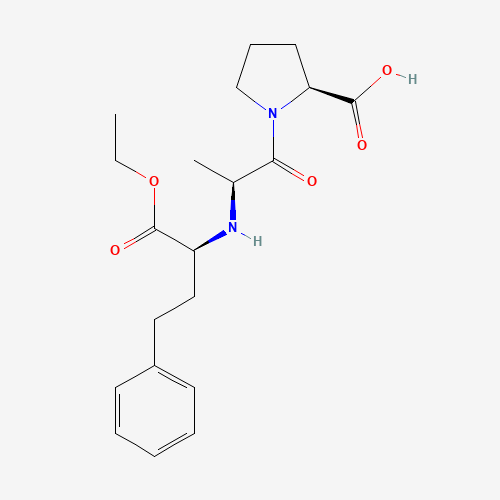
| Molecular Formula |
C20H28N2O5 |
| CAS Registry Number |
75847-73-3 |
| SMILES |
CCOC(=O)C(CCC1=CC=CC=C1)NC(C)C(=O)N2CCCC2C(=O)O |
| Chemical Structure |
|
|
| ADR Related Proteins Induced by Drug |
|
|
| ADRs Induced by Drug |
|
|

