| Pharmaceutical Information |
| Drug Name |
Zolpidem |
| Drug ID |
BADD_D02394 |
| Description |
Zolpidem, also known as _Ambien_, is a hypnotic drug that was initially approved by the FDA in 1992 [FDA label]. Zolpidem improves sleep in patients with insomnia. It is aimed for use in patients with difficulties initiating sleep. This drug decreases the time to fall asleep (sleep latency), increases the duration of sleep, and decreases the number of awakenings during sleep in patients with temporary (transient) insomnia. It is available in both immediate acting and extended release forms [FDA label], [F3802].
Its tolerability profile is favorable when administered according to the manufacturer’s instructions, with a low risk of drug withdrawal, drug dependence, and drug tolerance [A175426]. In addition, zolpidem improves sleep quality in patients suffering from chronic insomnia and can show mild muscle relaxant properties [L5584]. Research also shows that zolpidem is rapid and effective in restoring brain function for patients in a vegetative state following brain injury. This drug has the propensity to completely or partially reverse the abnormal metabolism of damaged brain cells after injury [L5584], [A175444]. |
| Indications and Usage |
This drug is indicated for the short-term treatment of insomnia in adults characterized by difficulties with sleep initiation [FDA label]. |
| Marketing Status |
approved |
| ATC Code |
N05CF02 |
| DrugBank ID |
DB00425
|
| KEGG ID |
D08690
|
| MeSH ID |
D000077334
|
| PubChem ID |
5732
|
| TTD Drug ID |
D0T1WN
|
| NDC Product Code |
Not Available |
| UNII |
7K383OQI23
|
| Synonyms |
Zolpidem | Imidazo(1,2-a)pyridine-3-acetamide, N,N,6-trimethyl-2-(4-methylphenyl)- | SL 80.0750 | SL-800750-23-N | SL 800750 23 N | Zolirin | Zolpi-Lich | Zolpi Lich | Zolpidem 1A Pharma | Zolpidem AbZ | Zolpidem Tartrate | N,N,6-Trimethyl-2-(4-methylphenyl)imidazo(1,2a)pyridine-3-acetamide hemitartrate | Zolpidem Hemitartrate | Zolpinox | Zolpimist | Ambien | Amsic | Bikalm | Stilnoct | Stilnox | Dalparan | Zodormdura | Zoldem |
|
| Chemical Information |
| Molecular Formula |
C19H21N3O |
| CAS Registry Number |
82626-48-0 |
| SMILES |
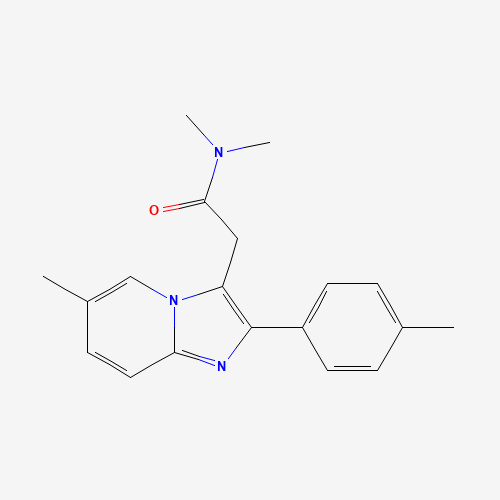
CC1=CC=C(C=C1)C2=C(N3C=C(C=CC3=N2)C)CC(=O)N(C)C |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

