| Pharmaceutical Information |
| Drug Name |
Verapamil |
| Drug ID |
BADD_D02348 |
| Description |
Verapamil is a phenylalkylamine calcium channel blocker used in the treatment of high blood pressure, heart arrhythmias, and angina,[L8791] and was the first calcium channel antagonist to be introduced into therapy in the early 1960s.[A188514] It is a member of the non-dihydropyridine class of calcium channel blockers, which includes drugs like [diltiazem] and [flunarizine], but is chemically unrelated to other cardioactive medications.[L8791] Verapamil is administered as a racemic mixture containing equal amounts of the S- and R-enantiomer, each of which is pharmacologically distinct - the S-enantiomer carries approximately 20-fold greater potency than the R-enantiomer, but is metabolized at a higher rate.[A188435] |
| Indications and Usage |
Verapamil is indicated in the treatment of vasopastic (i.e. Prinzmetal's) angina, unstable angina, and chronic stable angina. It is also indicated to treat hypertension, for the prophylaxis of repetitive paroxysmal supraventricular tachycardia, and in combination with digoxin to control ventricular rate in patients with atrial fibrillation or atrial flutter.[L8791] Given intravenously, it is indicated for the treatment of various supraventricular tachyarrhythmias, including rapid conversion to sinus rhythm in patients with supraventricular tachycardia and for temporary control of ventricular rate in patients with atrial fibrillation or atrial flutter.[L10481]
Verapamil is commonly used off-label for prophylaxis of cluster headaches.[A13983] |
| Marketing Status |
approved |
| ATC Code |
C08DA01 |
| DrugBank ID |
DB00661
|
| KEGG ID |
D02356
|
| MeSH ID |
D014700
|
| PubChem ID |
2520
|
| TTD Drug ID |
D0R0FE
|
| NDC Product Code |
70966-0029 |
| UNII |
CJ0O37KU29
|
| Synonyms |
Verapamil | Iproveratril | Cordilox | Dexverapamil | Verapamil Hydrochloride | Hydrochloride, Verapamil | Finoptin | Izoptin | Isoptine | Isoptin | Lekoptin | Calan | Falicard |
|
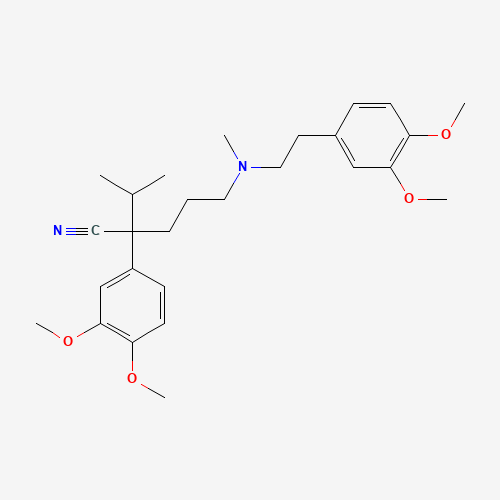
| Chemical Information |
| Molecular Formula |
C27H38N2O4 |
| CAS Registry Number |
52-53-9 |
| SMILES |
CC(C)C(CCCN(C)CCC1=CC(=C(C=C1)OC)OC)(C#N)C2=CC(=C(C=C2)OC)OC |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

