| Pharmaceutical Information |
| Drug Name |
Vemurafenib |
| Drug ID |
BADD_D02344 |
| Description |
Vemurafenib is a competitive kinase inhibitor with activity against BRAF kinase with mutations like V600E.[A31269] It exerts its function by binding to the ATP-binding domain of the mutant BRAF.[A31270] Vemurafenib was co-developed by Roche and Plexxikon and it obtained its FDA approval on August 17, 2011, under the company Hoffmann La Roche. After approval, Roche in collaboration with Genentech launched a broad development program. [L1012] |
| Indications and Usage |
Vemurafenib is approved since 2011 for the treatment of metastatic melanoma with a mutation on BRAF in the valine located in the exon 15 at codon 600, this mutation is denominated as V600E.[A31270] The V600E mutation, a substitution of glutamic acid for valine, accounts for 54% of the cases of cutaneous melanoma.[A31271]
Vemurafenib approval was extended in 2017, for its use as a treatment of adult patients with Erdheim-Chester Disease whose cancer cells present BRAF V600 mutation.[L1013] Erdheim-Chester disease is an extremely rare histiocyte cell disorder that affects large bones, large vessels, central nervous system, as well as, skin and lungs. It is reported an association of Erdheim-Chester disease and V600E mutation.[A31272] |
| Marketing Status |
approved |
| ATC Code |
L01EC01 |
| DrugBank ID |
DB08881
|
| KEGG ID |
D09996
|
| MeSH ID |
D000077484
|
| PubChem ID |
42611257
|
| TTD Drug ID |
D0Y9EW
|
| NDC Product Code |
50242-090 |
| UNII |
207SMY3FQT
|
| Synonyms |
Vemurafenib | PLX4032 | PLX 4032 | RG7204 | RG-7204 | RG 7204 | Zelboraf | R05185426 |
|
| Chemical Information |
| Molecular Formula |
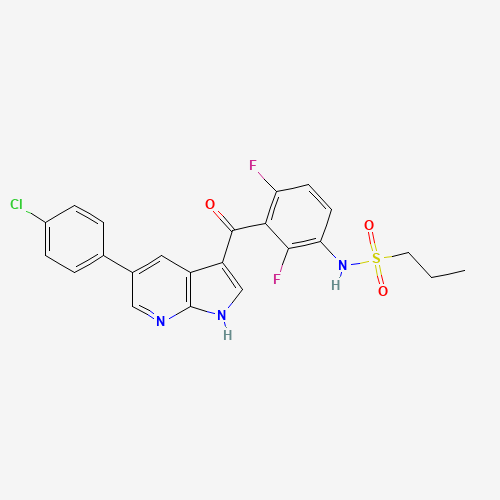
C23H18ClF2N3O3S |
| CAS Registry Number |
918504-65-1 |
| SMILES |
CCCS(=O)(=O)NC1=C(C(=C(C=C1)F)C(=O)C2=CNC3=C2C=C(C=N3)C4=CC=C(C=C4)Cl)F |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

