| Pharmaceutical Information |
| Drug Name |
Trimethoprim |
| Drug ID |
BADD_D02290 |
| Description |
Trimethoprim is an antifolate antibacterial agent that inhibits bacterial dihydrofolate reductase (DHFR), a critical enzyme that catalyzes the formation of tetrahydrofolic acid (THF) - in doing so, it prevents the synthesis of bacterial DNA and ultimately continued bacterial survival.[L11893] Trimethoprim is often used in combination with [sulfamethoxazole] due to their complementary and synergistic mechanisms but may be used as a monotherapy in the treatment and/or prophylaxis of urinary tract infections.[L11893,L11830] It is structurally and chemically related to [pyrimethamine], another antifolate antimicrobial used in the treatment of plasmodial infections.[T707] |
| Indications and Usage |
As a monotherapy, trimethoprim is indicated for the treatment of acute episodes of uncomplicated urinary tract infections caused by susceptible bacteria, including _E. coli._, _K. pneumoniae_, _Enterobacter spp._, _P. mirabilis_, and coagulase-negative _Staphylococcus_ species.[L11893,L11890] In various formulations in combination with [sulfamethoxazole], trimethoprim is indicated for the following infections caused by bacteria with documented susceptibility: urinary tract infections, acute otitis media in pediatric patients (when clinically indicated), acute exacerbations of chronic bronchitis in adults, enteritis caused by susceptible _Shigella_, prophylaxis and treatment of _Pneumocystis jiroveci_ pneumonia, and travelers' diarrhea caused by enterotoxigenic _E. coli_.[L11830,L11863]
Trimethoprim is available as an ophthalmic solution in combination with [polymyxin B] for the treatment of acute bacterial conjunctivitis, blepharitis, and blepharoconjunctivitis caused by susceptible bacteria.[L11887] |
| Marketing Status |
approved; vet_approved |
| ATC Code |
J01EA01 |
| DrugBank ID |
DB00440
|
| KEGG ID |
D00145
|
| MeSH ID |
D014295
|
| PubChem ID |
5578
|
| TTD Drug ID |
D0AO5H
|
| NDC Product Code |
64374-001; 43386-330; 38779-0770; 65863-0008; 49452-7925; 40032-330; 60592-716; 62991-3173; 70954-541; 51927-1693; 51862-486; 57451-1111; 57451-1202; 65863-0004; 62991-2072; 72672-001; 51407-738 |
| UNII |
AN164J8Y0X
|
| Synonyms |
Trimethoprim | Trimpex | Proloprim |
|
| Chemical Information |
| Molecular Formula |
C14H18N4O3 |
| CAS Registry Number |
738-70-5 |
| SMILES |
COC1=CC(=CC(=C1OC)OC)CC2=CN=C(N=C2N)N |
| Chemical Structure |
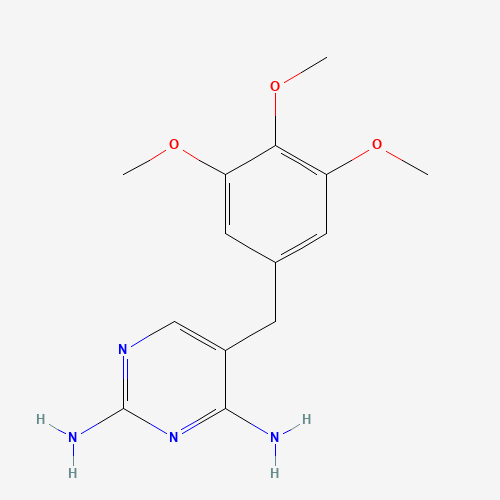
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

