| Pharmaceutical Information |
| Drug Name |
Teniposide |
| Drug ID |
BADD_D02153 |
| Description |
Teniposide is a semisynthetic derivative of podophyllotoxin that exhibits antitumor activity. Teniposide inhibits DNA synthesis by forming a complex with topoisomerase II and DNA. This complex induces breaks in double stranded DNA and prevents repair by topoisomerase II binding. Accumulated breaks in DNA prevent cells from entering into the mitotic phase of the cell cycle, and lead to cell death. Teniposide acts primarily in the G2 and S phases of the cycle. |
| Indications and Usage |
Teniposide is used for the treatment of refractory acute lymphoblastic leukaemia |
| Marketing Status |
approved |
| ATC Code |
L01CB02 |
| DrugBank ID |
DB00444
|
| KEGG ID |
D02698
|
| MeSH ID |
D013713
|
| PubChem ID |
5396
|
| TTD Drug ID |
D01DBQ
|
| NDC Product Code |
Not Available |
| UNII |
957E6438QA
|
| Synonyms |
Teniposide | Demethyl Epipodophyllotoxin Thenylidine Glucoside | Vumon | VM-26 | VM 26 | VM26 | Teniposide, (5a alpha,9 alpha(S*))-Isomer | NSC-122819 | NSC 122819 | NSC122819 |
|
| Chemical Information |
| Molecular Formula |
C32H32O13S |
| CAS Registry Number |
29767-20-2 |
| SMILES |
COC1=CC(=CC(=C1O)OC)C2C3C(COC3=O)C(C4=CC5=C(C=C24)OCO5)OC6C(C(C7C(O6)COC(O7)C8=C
C=CS8)O)O |
| Chemical Structure |
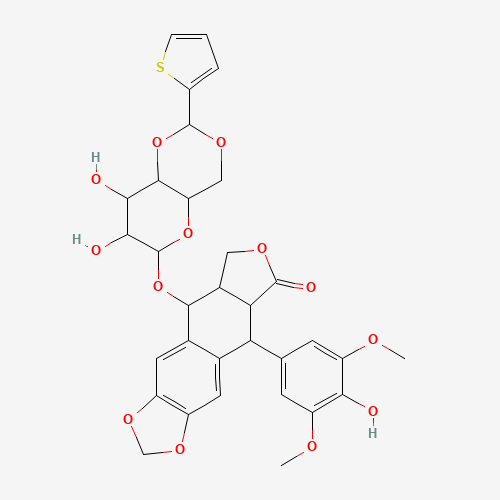
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

