| Pharmaceutical Information |
| Drug Name |
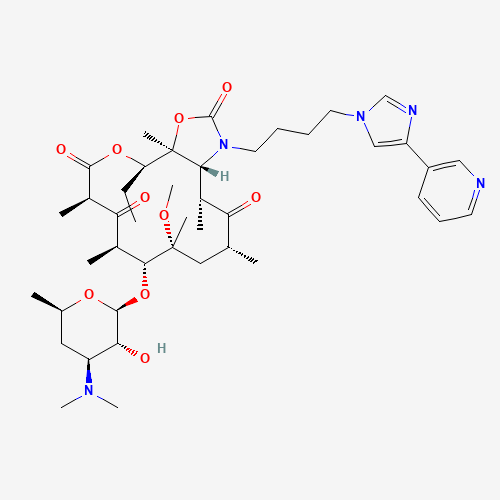
Telithromycin |
| Drug ID |
BADD_D02146 |
| Description |
Telithromycin, a semi-synthetic erythromycin derivative, belongs to a new chemical class of antibiotics called ketolides. Ketolides have been recently added to the macrolide-lincosamide-streptogramin class of antibiotics. Similar to the macrolide antibiotics, telithromycin prevents bacterial growth by interfering with bacterial protein synthesis. Telithromycin binds to the 50S subunit of the 70S bacterial ribosome and blocks further peptide elongation. Binding occurs simultaneously at to two domains of 23S RNA of the 50S ribosomal subunit, domain II and V, where older macrolides bind only to one. It is used to treat mild to moderate respiratory infections. |
| Indications and Usage |
For the treatment of Pneumococcal infection, acute sinusitis, acute bacterial tonsillitis, acute bronchitis and bronchiolitis, lower respiratory tract infection and lobar (pneumococcal) pneumonia. |
| Marketing Status |
approved |
| ATC Code |
J01FA15 |
| DrugBank ID |
DB00976
|
| KEGG ID |
D01078
|
| MeSH ID |
C106791
|
| PubChem ID |
3002190
|
| TTD Drug ID |
D09HNR
|
| NDC Product Code |
Not Available |
| UNII |
KI8H7H19WL
|
| Synonyms |
telithromycin | Ketek | RU 66647 | RU-66647 | HMR 3647 | HMR3647 | HMR-3647 |
|
| Chemical Information |
| Molecular Formula |
C43H65N5O10 |
| CAS Registry Number |
191114-48-4 |
| SMILES |
CCC1C2(C(C(C(=O)C(CC(C(C(C(=O)C(C(=O)O1)C)C)OC3C(C(CC(O3)C)N(C)C)O)(C)OC)C)C)N(C
(=O)O2)CCCCN4C=C(N=C4)C5=CN=CC=C5)C |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

