| Pharmaceutical Information |
| Drug Name |
Protirelin |
| Drug ID |
BADD_D01871 |
| Description |
Protirelin is the pharmaceutically available synthetic analogue of the endogenous peptide thyrotropin-releasing hormone (TRH). It is a tri-peptide tropic hormone, released by the hypothalamus, that stimulates the release of Thyroid Stimulating Hormone (TSH) and prolactin from the anterior pituitary.
Although not currently available in any FDA-approved product, protirelin is a component of the TRH Test where it is used to test the response of the anterior pituitary gland in conditions such as secondary hypothyroidism and acromegaly. |
| Indications and Usage |
Not Available |
| Marketing Status |
approved; investigational |
| ATC Code |
V04CJ02 |
| DrugBank ID |
DB09421
|
| KEGG ID |
D00176
|
| MeSH ID |
D013973
|
| PubChem ID |
638678
|
| TTD Drug ID |
D08BTB
|
| NDC Product Code |
Not Available |
| UNII |
5Y5F15120W
|
| Synonyms |
Thyrotropin-Releasing Hormone | Thyrotropin Releasing Hormone | Thyrotropin-Releasing Factor | Thyrotropin Releasing Factor | Protirelin | Antepan | Protirelin Tartrate (1:1) | Proterelin Tartrate | Thyrotropin-Releasing Hormone Tartrate | Thyrotropin Releasing Hormone Tartrate | Relefact TRH | TRH, Relefact | Thypinone | Thyroliberin | Thyroliberin TRH Merck | TRH Prem | Prem, TRH | Proterelin Tartrate Hydrate | Hydrate, Proterelin Tartrate | Tartrate Hydrate, Proterelin | Abbott-38579 | Abbott 38579 | Abbott38579 | Stimu-TSH | Stimu TSH | StimuTSH | TRH Ferring |
|
| Chemical Information |
| Molecular Formula |
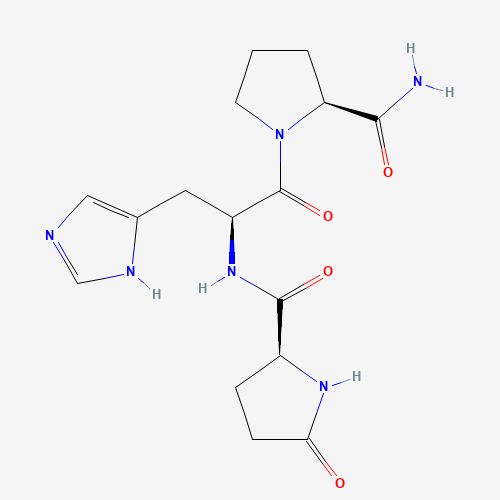
C16H22N6O4 |
| CAS Registry Number |
24305-27-9 |
| SMILES |
C1CC(N(C1)C(=O)C(CC2=CN=CN2)NC(=O)C3CCC(=O)N3)C(=O)N |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

