| Pharmaceutical Information |
| Drug Name |
Erythropoietin |
| Drug ID |
BADD_D00808 |
| Description |
Erythropoietin (EPO) is a growth factor produced in the kidneys that stimulates the production of red blood cells. It works by promoting the division and differentiation of committed erythroid progenitors in the bone marrow [FDA Label]. Epoetin alfa (Epoge) was developed by Amgen Inc. in 1983 as the first rhEPO commercialized in the United States, followed by other alfa and beta formulations. Epoetin alfa is a 165-amino acid erythropoiesis-stimulating glycoprotein produced in cell culture using recombinant DNA technology and is used for the treatment of patients with anemia associated with various clinical conditions, such as chronic renal failure, antiviral drug therapy, chemotherapy, or a high risk for perioperative blood loss from surgical procedures [FDA Label]. It has a molecular weight of approximately 30,400 daltons and is produced by mammalian cells into which the human erythropoietin gene has been introduced. The product contains the identical amino acid sequence of isolated natural erythropoietin and has the same biological activity as the endogenous erythropoietin. Epoetin alfa biosimilar, such as Retacrit (epoetin alfa-epbx or epoetin zeta), has been formulated to allow more access to treatment options for patients in the market [L2784]. The biosimilar is approved by the FDA and EMA as a safe, effective and affordable biological product and displays equivalent clinical efficacy, potency, and purity to the reference product [A7504]. Epoetin alfa formulations can be administered intravenously or subcutaneously. |
| Indications and Usage |
Indicated in adult and paediatric patients for the:
- treatment of anemia due to Chronic Kidney Disease (CKD) in patients on dialysis and not on dialysis.
- treatment of anemia due to zidovudine in patients with HIV-infection.
- treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy, and upon initiation, there is a minimum of two additional months of planned chemotherapy.
- reduction of allogeneic RBC transfusions in patients undergoing elective, noncardiac, nonvascular surgery. |
| Marketing Status |
approved |
| ATC Code |
B03XA01 |
| DrugBank ID |
DB00016
|
| KEGG ID |
Not Available
|
| MeSH ID |
D004921
|
| PubChem ID |
11751549
|
| TTD Drug ID |
D0G5JW
|
| NDC Product Code |
63552-138; 59676-340; 59676-303; 63552-139; 59676-302; 59676-304; 63552-140; 63552-135; 63552-136; 63552-137; 63552-141; 59676-310; 59676-312; 59676-320 |
| UNII |
64FS3BFH5W
|
| Synonyms |
Erythropoietin |
|
| Chemical Information |
| Molecular Formula |
C22H22ClKN6O |
| CAS Registry Number |
124750-99-8 |
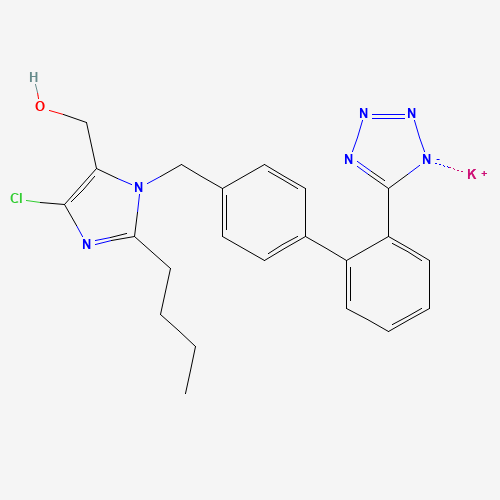
| SMILES |
CCCCC1=NC(=C(N1CC2=CC=C(C=C2)C3=CC=CC=C3C4=NN=N[N-]4)CO)Cl.[K+] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

