| Pharmaceutical Information |
| Drug Name |
Semaglutide |
| Drug ID |
BADD_D02518 |
| Description |
Semaglutide is a glucagon-like peptide 1 (GLP-1) analog used to manage type 2 diabetes along with lifestyle changes, such as dietary restrictions and increased physical activity.[A31421,L8681] Other members of this drug class include [Exenatide] and [Liraglutide]. Semaglutide was developed by Novo Nordisk and approved by the FDA for subcutaneous injection in December 2017.[L8681] The tablet formulation was approved for oral administration in September 2019. Semaglutide works by binding to and activating the GLP-1 receptor, thereby stimulating insulin secretion and reducing blood glucose.[L8678]
The subcutaneous injection is administered once weekly and the tablet is administered once a day. Semaglutide offers a competitive advantage over other drugs used to manage diabetes, which may require several daily doses. Clinical trials have determined that this drug reduces glycosylated hemoglobin (HbA1c) levels and reduces body weight, proving to be effective for patients with type 2 diabetes.[A186053] In June 2021, semaglutide was approved by the FDA for chronic weight management in adults with general obesity or overweight who have at least one weight-related condition: this marks semaglutide as the first approved drug for such use since 2014.[L34485] Health Canada also approved semaglutide in November 2021 for the treatment of adults with obesity.[L39347] |
| Indications and Usage |
Semaglutide is indicated to improve glycemic control in adults with type 2 diabetes mellitus as an adjunct of diet and exercise. The approved therapeutic doses are 0.5 mg and 1 mg.[L1068] Diabetes mellitus type 2 is a long-term metabolic disorder characterized by high blood sugar, insulin resistance and lack of insulin. Its onset is determined by the loss ability of beta cells to respond to an increased plasma glucose. This disease is predominantly caused by lifestyle factors like overweight and obesity. The key feature on type 2 diabetes is the presence of insulin resistance which reduced the capacity of insulin to exert its functions at normal at any given concentration. The secretion of insulin is stimulated by the action of incretins in the gut like glucagon-like peptide 1, which also delays gastric emptying and induces satiety, and glucose-dependent insulinotropic polypeptide.[T59] |
| Marketing Status |
Not Available |
| ATC Code |
A10BJ06 |
| DrugBank ID |
DB13928
|
| KEGG ID |
D10025
|
| MeSH ID |
C000591245
|
| PubChem ID |
56843331
|
| TTD Drug ID |
D02ULU
|
| NDC Product Code |
41524-0012; 71796-039; 0169-4136; 0169-4517; 43835-0028; 73569-024; 43835-0030; 0169-4501; 70518-2143; 43835-0029; 50090-5824; 0169-4525; 0169-4524; 73212-030; 50090-5138; 0420-9008; 0169-4130; 0169-4505; 50090-5139; 0169-4132 |
| Synonyms |
semaglutide | rybelsus | Ozempic |
|
| Chemical Information |
| Molecular Formula |
C187H291N45O59 |
| CAS Registry Number |
910463-68-2 |
| SMILES |
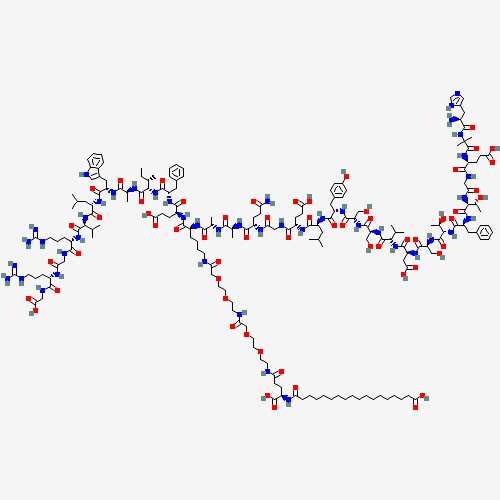
CCC(C)C(C(=O)NC(C)C(=O)NC(CC1=CNC2=CC=CC=C21)C(=O)NC(CC(C)C)C(=O)NC(C(C)C)C(=O)N
C(CCCNC(=N)N)C(=O)NCC(=O)NC(CCCNC(=N)N)C(=O)NCC(=O)O)NC(=O)C(CC3=CC=CC=C3)NC(=O)
C(CCC(=O)O)NC(=O)C(CCCCNC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CCC(C(=O)O)NC(=O)CCCCCCCC
CCCCCCCCC(=O)O)NC(=O)C(C)NC(=O)C(C)NC(=O)C(CCC(=O)N)NC(=O)CNC(=O)C(CCC(=O)O)NC(=
O)C(CC(C)C)NC(=O)C(CC4=CC=C(C=C4)O)NC(=O)C(CO)NC(=O)C(CO)NC(=O)C(C(C)C)NC(=O)C(C
C(=O)O)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C(CC5=CC=CC=C5)NC(=O)C(C(C)O)NC(=O)CNC(=O)
C(CCC(=O)O)NC(=O)C(C)(C)NC(=O)C(CC6=CN=CN6)N |
| Chemical Structure |
|
|
| ADR Related Proteins Induced by Drug |
| ADR Term |
Protein Name |
UniProt AC |
TTD Target ID |
PMID |
| Not Available | Not Available | Not Available | Not Available | Not Available |
|
| ADRs Induced by Drug |
|
|

