| Pharmaceutical Information |
| Drug Name |
Enasidenib |
| Drug ID |
BADD_D02517 |
| Description |
Enasidenib is an orally available treatment for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with specific mutations in the isocitrate dehydrogenase 2 (IDH2) gene, which is a recurrent mutation detected in 12-20% of adult patients with AML [A20344, A20345]. Patients eligible for this treatment are selected by testing the presence of IDH2 mutations in the blood or bone marrow. This small molecule acts as an allosteric inhibitor of mutant IDH2 enzyme to prevent cell growth, and it also has shown to block several other enzymes that play a role in abnormal cell differentiation. First developed by Agios Pharmaceuticals and licensed to Celgene, enasidenib was approved by U.S. Food and Drug Administration on August 1, 2017. |
| Indications and Usage |
Indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with an isocitrate dehydrogenase-2 (IDH2) mutation. |
| Marketing Status |
approved; investigational |
| ATC Code |
L01XX59 |
| DrugBank ID |
DB13874
|
| KEGG ID |
D10901
|
| MeSH ID |
C000605269
|
| PubChem ID |
89683805
|
| TTD Drug ID |
D0K7FT
|
| NDC Product Code |
Not Available |
| UNII |
3T1SS4E7AG
|
| Synonyms |
enasidenib | AG-221 | Idhifa |
|
| Chemical Information |
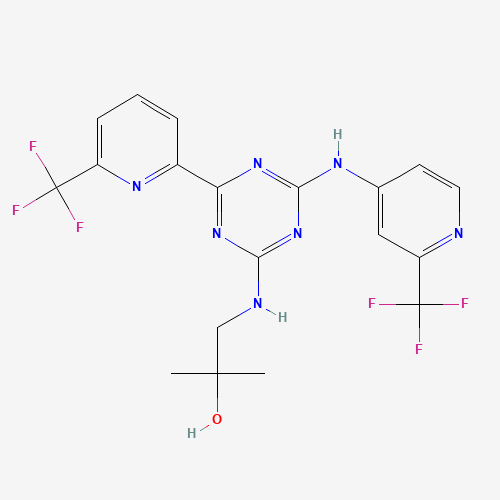
| Molecular Formula |
C19H17F6N7O |
| CAS Registry Number |
1446502-11-9 |
| SMILES |
CC(C)(CNC1=NC(=NC(=N1)C2=NC(=CC=C2)C(F)(F)F)NC3=CC(=NC=C3)C(F)(F)F)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

