| Pharmaceutical Information |
| Drug Name |
Neratinib |
| Drug ID |
BADD_D02489 |
| Description |
Neratinib was approved in July 2017 for use as an extended adjuvant therapy in Human Epidermal Growth Factor Receptor 2 (HER2) positive breast cancer. Approval was granted to Puma Biotechnology Inc. for the tradename Nerlynx. Neratinib is currently under investigation for use in many other forms of cancer. |
| Indications and Usage |
For use as an extended adjuvant treatment in adult patients with early stage HER2-overexpressed/amplified breast cancer, to follow adjuvant trastuzumab-based therapy [FDA Label]. |
| Marketing Status |
Not Available |
| ATC Code |
L01EH02 |
| DrugBank ID |
DB11828
|
| KEGG ID |
D08950
|
| MeSH ID |
C487932
|
| PubChem ID |
9915743
|
| TTD Drug ID |
D0U1ZV
|
| NDC Product Code |
70437-240 |
| Synonyms |
neratinib | N-(4-(3-chloro-4-(2-pyridinylmethoxy)anilino)-3-cyano-7-ethoxy-6-quinolyl)-4-(dimethylamino)-2-butenamide | HKI 272 | HKI272 | HKI-272 | neratinib maleate | Nerlynx |
|
| Chemical Information |
| Molecular Formula |
C30H29ClN6O3 |
| CAS Registry Number |
698387-09-6 |
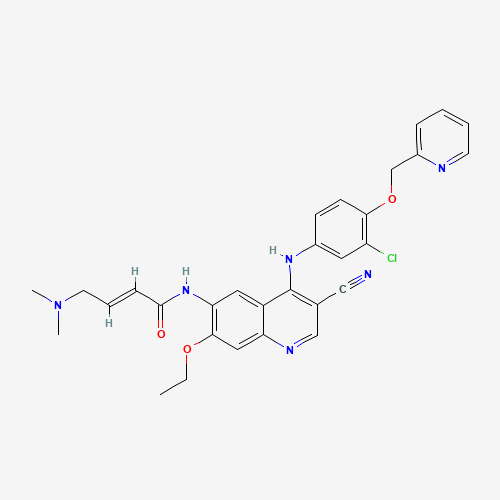
| SMILES |
CCOC1=C(C=C2C(=C1)N=CC(=C2NC3=CC(=C(C=C3)OCC4=CC=CC=N4)Cl)C#N)NC(=O)C=CCN(C)C |
| Chemical Structure |
|
|
| ADR Related Proteins Induced by Drug |
| ADR Term |
Protein Name |
UniProt AC |
TTD Target ID |
PMID |
| Not Available | Not Available | Not Available | Not Available | Not Available |
|
| ADRs Induced by Drug |
|
|

