| Pharmaceutical Information |
| Drug Name |
Lanreotide |
| Drug ID |
BADD_D02461 |
| Description |
Lanreotide is a drug employed in the management of acromegaly (a hormonal condition caused by excess growth hormone) in addition to symptoms caused by neuroendocrine tumors, especially carcinoid syndrome. This drug is a long-acting analog of the drug somatostatin, a growth hormone inhibitor. Lanreotide is manufactured by the company, _Ipsen Pharmaceuticals_ as lanreotide acetate, and marketed as _Somatuline_. It is approved in several countries worldwide, including the United Kingdom, Australia, and Canada. Lanreotide was first approved for use in the United States by the FDA on August 30, 2007. |
| Indications and Usage |
Lanreotide is a somatostatin analog approved for treatment of neuroendocrine tumours and acromegaly. (2) |
| Marketing Status |
Not Available |
| ATC Code |
H01CB03 |
| DrugBank ID |
DB06791
|
| KEGG ID |
D04666
|
| MeSH ID |
C060347
|
| PubChem ID |
6918011
|
| TTD Drug ID |
D0M2YE
|
| NDC Product Code |
52416-117 |
| Synonyms |
lanreotide | 3-(2-naphthyl)alanyl-cystinyl-tyrosyl-tryptophyl-lysyl-valyl-cystinyl-threonine amide | D-Nal-Cys-Tyr-Trp-Lys-Val-Cys-Thr-NH2 | angiopeptin | naphthalenyl-cyclo(cysteinyl-tyrosyl-tryptophyl-lysyl-valyl-cysteinyl)threoninamide | L-Threoninamide, 3-(2-naphthalenyl)-D-alanyl-L-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-valyl-L-cysteinyl-, cyclic (2-7)-disulfide | Nal-cyclo(Cys-Tyr-Trp-Lys-Val-Cys)-Thr-NH2 | Nal-cyclo(Cys-Tyr-Trp-Lys-Val-Cys)Thr-NH2 | 2-naphthylalanyl-cyclo(cysteinyl-tyrosyl-tryptophyl-lysyl-valyl-cysteinyl)-threoninamide | 3-(2-naphthyl)-D-Ala-Cys-Tyr-D-Trp-Lys-Val-Cys-Thr-NH2 | naphthyl-cyclo(Cys-Tyr-Trp-Lys-Val-Cys)Thr-NH2 | BIM 23014 | BIM-23014 | DC 13-116 | DC13-116 | DC-13-116 | lanreotide acetate | Somatulina | Somatuline | lanreotide-SR | BIM 23014 C | BIM 23014C | BIM-23014 C | 188Re-lanreotide | Somatulin |
|
| Chemical Information |
| Molecular Formula |
C54H69N11O10S2 |
| CAS Registry Number |
108736-35-2 |
| SMILES |
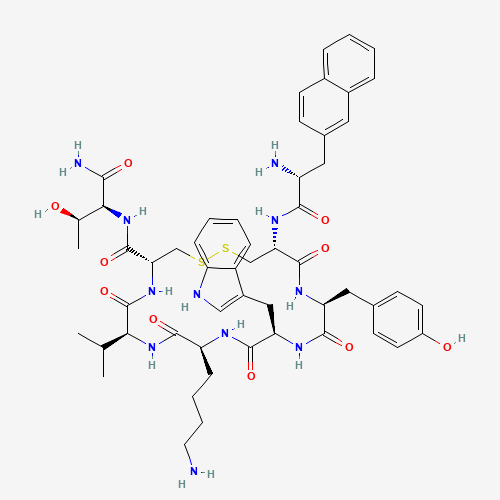
CC(C)C1C(=O)NC(CSSCC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N1)CCCCN)CC2=CNC3=CC=CC=C32)CC
4=CC=C(C=C4)O)NC(=O)C(CC5=CC6=CC=CC=C6C=C5)N)C(=O)NC(C(C)O)C(=O)N |
| Chemical Structure |
|
|
| ADR Related Proteins Induced by Drug |
| ADR Term |
Protein Name |
UniProt AC |
TTD Target ID |
PMID |
| Not Available | Not Available | Not Available | Not Available | Not Available |
|
| ADRs Induced by Drug |
|
|

