| Pharmaceutical Information |
| Drug Name |
Lisdexamfetamine |
| Drug ID |
BADD_D02440 |
| Description |
Also known as _Vyvanse_, lisdexamfetamine (L-lysine-d-amphetamine) is a prodrug of the psychostimulant d-amphetamine [A40246]. It is paired with the essential amino acid _L-lysine_. Lisdexamfetamine dimesylate increases attention span and decreases restlessness in children and adults who are overactive/hyperactive, cannot concentrate for long periods, or are easily distracted or impulsive [A2230].
As a central nervous system stimulant, lisdexamfetamine is utilized as an adjunct therapy in the treatment of attention deficit hyperactivity disorder (ADHD). As a prodrug, lisdexamfetamine was specifically engineered as an abuse-resistant product [F2368]. The mechanism by which this occurs is through delayed release after ingestion (unlike some other psychostimulant drugs, which may be abused). After oral administration and absorption, enzyme hydrolysis after contact with red blood cells metabolize lisdexamfetamine into L- lysine, a naturally occurring essential amino acid and active _d-amphetamine_, which is responsible for the drug’s pharmacological effects. Gastrointestinal pH does not affect this conversion, and the addition of the L-lysine slows the amount of d-amphetamine available in the circulation and central nervous system [F2368]. |
| Indications and Usage |
For the treatment of Attention-deficit/hyperactivity disorder (ADHD) and for moderate to severe binge eating disorder in adults [FDA label], [A40246].
This drug is not indicated for weight loss. Use of other sympathomimetic drugs for weight loss is associated with serious cardiovascular effects. The safety and effectiveness of this drug for the treatment of obesity have not yet been determined [FDA label].
|
| Marketing Status |
approved; investigational |
| ATC Code |
N06BA12 |
| DrugBank ID |
DB01255
|
| KEGG ID |
D08130
|
| MeSH ID |
D000069478
|
| PubChem ID |
11597698
|
| TTD Drug ID |
D00DEF
|
| NDC Product Code |
Not Available |
| UNII |
H645GUL8KJ
|
| Synonyms |
Lisdexamfetamine Dimesylate | Dimesylate, Lisdexamfetamine | Lis-dexamfetamine Dimesylate | Dimesylate, Lis-dexamfetamine | Lis dexamfetamine Dimesylate | Vyvanse | Elvanse | Lisdexamfetamine | NRP104 | NRP-104 | NRP 104 |
|
| Chemical Information |
| Molecular Formula |
C15H25N3O |
| CAS Registry Number |
608137-32-2 |
| SMILES |
CC(CC1=CC=CC=C1)NC(=O)C(CCCCN)N |
| Chemical Structure |
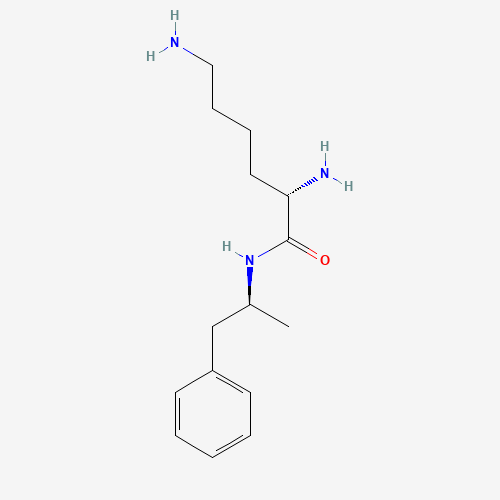
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

