| Pharmaceutical Information |
| Drug Name |
Vinorelbine |
| Drug ID |
BADD_D02363 |
| Description |
Vinorelbine is an anti-mitotic chemotherapy drug that is used in the treatment of several types of malignancies, including breast cancer and non-small cell lung cancer (NSCLC) [L1998]. It was initially approved in the USA in 1990's for the treatment of NSCLC [L2010].
It is a third-generation vinca alkaloid. The introduction of third-generation drugs (vinorelbine, gemcitabine, taxanes) in platinum combination improved survival of patients with advanced NSCLC, with very similar results from the various drugs. Treatment toxicities are considerable in the combination treatment setting [A32347].
A study was done on the clearance rate of vinorelbine on individuals with various single polymorphonuclear mutations. It was found that there was 4.3-fold variation in vinorelbine clearance across the cohort, suggesting a strong influence of genetics on the clearance of this drug [L2002]. |
| Indications and Usage |
Vinorelbine tartrate is indicated for adults in the treatment of advanced non-small cell lung cancer (NSCLC), as a single therapy or in combination with other chemotherapeutic drugs [L1998].
Used in relapsed or refractory Hodgkin lymphoma, in combination with other chemotherapy agents [L2011].
For the treatment of desmoid tumor or aggressive fibromatosis, in combination with methotrexate [L2011].
For the treatment of recurrent or metastatic squamous cell head and neck cancer [L2011].
For the treatment of recurrent ovarian cancer [L2011].
For the treatment of metastatic breast cancer, in patients previously treated with anthracyline and/or taxane therapy [L2011].
For the treatment of HER2-positive, trastuzumab-resistant, advanced breast cancer in patients previously treated with a taxane, in combination with trastuzumab and everolimus [L2011].
|
| Marketing Status |
approved; investigational |
| ATC Code |
L01CA04 |
| DrugBank ID |
DB00361
|
| KEGG ID |
D08680
|
| MeSH ID |
D000077235
|
| PubChem ID |
5311497
|
| TTD Drug ID |
D01HTL
|
| NDC Product Code |
45963-607; 25021-204 |
| UNII |
Q6C979R91Y
|
| Synonyms |
Vinorelbine | 5'-Nor-anhydrovinblastine | 5' Nor anhydrovinblastine | KW 2307 | KW-2307 | KW2307 | Navelbine | Vinorelbine Tartrate |
|
| Chemical Information |
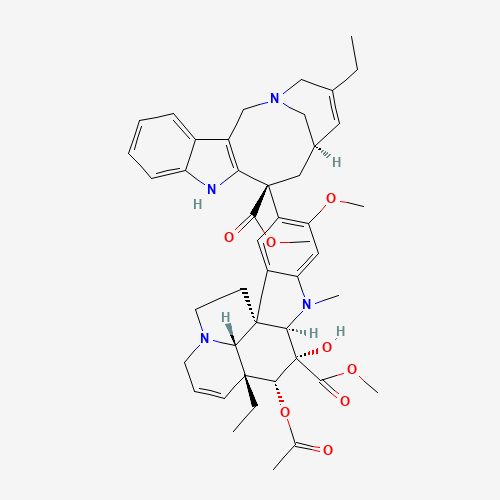
| Molecular Formula |
C45H54N4O8 |
| CAS Registry Number |
71486-22-1 |
| SMILES |
CCC1=CC2CC(C3=C(CN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N
6C)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

