| Pharmaceutical Information |
| Drug Name |
Tamoxifen |
| Drug ID |
BADD_D02111 |
| Description |
Tamoxifen is a non-steroidal antiestrogen used to treat estrogen receptor positive breast cancers as well as prevent the incidence of breast cancer in high risk populations.[A1025,L7799,L7802] Tamoxifen is used alone or as an adjuvant in these treatments.[L7799,L7802] Tamoxifen may no longer be the preferred treatment for these types of cancers as patients generally have better survival, side effect profiles, and compliance with [anastrozole].[A1026]
Tamoxifen was granted FDA approval on 30 December 1977.[L7799] |
| Indications and Usage |
Tamoxifen is indicated to treat estrogen receptor positive metastatic breast cancer in adults, as an adjuvant in the treatment of early stage estrogen receptor positive breast cancer in adults, to reduce the risk of invasive breast cancer after surgery and radiation in adult women with ductal carcinoma in situ.[L7802] |
| Marketing Status |
approved |
| ATC Code |
L02BA01 |
| DrugBank ID |
DB00675
|
| KEGG ID |
D08559
|
| MeSH ID |
D013629
|
| PubChem ID |
2733526
|
| TTD Drug ID |
D07KSG
|
| NDC Product Code |
Not Available |
| UNII |
094ZI81Y45
|
| Synonyms |
Tamoxifen | ICI-47699 | ICI 47699 | ICI47699 | Nolvadex | Novaldex | Tamoxifen Citrate | Citrate, Tamoxifen | Tomaxithen | Zitazonium | ICI-46474 | ICI 46474 | ICI46474 | ICI-46,474 | ICI 46,474 | ICI46,474 | Soltamox |
|
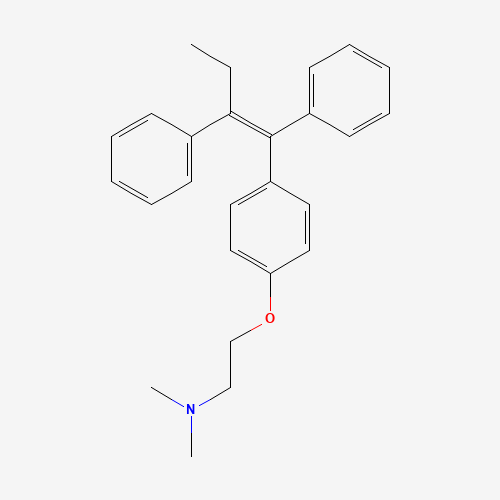
| Chemical Information |
| Molecular Formula |
C26H29NO |
| CAS Registry Number |
10540-29-1 |
| SMILES |
CCC(=C(C1=CC=CC=C1)C2=CC=C(C=C2)OCCN(C)C)C3=CC=CC=C3 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

