| Pharmaceutical Information |
| Drug Name |
Panobinostat lactate |
| Drug ID |
BADD_D01667 |
| Description |
Panobinostat is an oral deacetylace (DAC) inhibitor approved on February 23, 2015 by the FDA for the treatment of multiple myeloma. The approval was accelerated based on progression-free survival, therefore confirmatory trials by the sponsor to demonstrate clinical efficacy in multiple myeloma treatment are in progress of being conducted. Panobinostat is marketed by Novartis under the brand name Farydak. Panobinostat acts as a non-selective histone deacetylase inhibitor (pan-HDAC inhibitor) and it is the most potent DAC inhibiting agent available on the market. |
| Indications and Usage |
Panobinostat is indicated in the treatment of multiple myeloma in combination with dexamethasone and bortezomib in patients who have received 2 previous treatment regimens including bortezomib and an immunomodulatory agent. This indication is approved by accelerated approval based on progression free survival as of February 23, 2015. |
| Marketing Status |
approved; investigational |
| ATC Code |
L01XH03 |
| DrugBank ID |
DB06603
|
| KEGG ID |
D10019
|
| MeSH ID |
D000077767
|
| PubChem ID |
23725423
|
| TTD Drug ID |
D0E3SH
|
| NDC Product Code |
54893-0073 |
| UNII |
HN0T99OO4V
|
| Synonyms |
Panobinostat | LBH589 | NVP-LBH589 | NVP LBH589 | LBH 589 | Farydak |
|
| Chemical Information |
| Molecular Formula |
C24H29N3O5 |
| CAS Registry Number |
960055-56-5 |
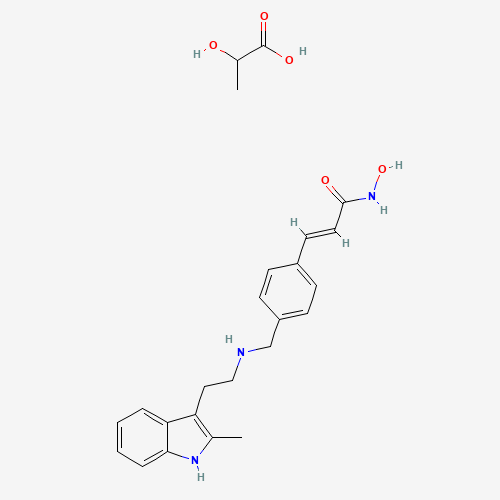
| SMILES |
CC1=C(C2=CC=CC=C2N1)CCNCC3=CC=C(C=C3)C=CC(=O)NO.CC(C(=O)O)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

