| Pharmaceutical Information |
| Drug Name |
Ketotifen |
| Drug ID |
BADD_D01234 |
| Description |
Ketotifen is a benzocycloheptathiophene derivative[A231204] with potent antihistaminic and mast cell stabilizing properties. It has a similar structure to some other first-generation antihistamines such as [cyproheptadine] and [azatadine].[A231204]
Ketotifen was first developed in Switzerland in 1970 by Sandoz Pharmaceuticals and was initially marketed for the treatment of anaphylaxis.[A231204] In the US, it is now used in an over-the-counter ophthalmic formulation for the treatment of itchy eyes associated with allergies,[L32278] and in Canada a prescription-only oral formulation is available and indicated as an add-on therapy for children with atopic asthma.[L32283] In addition, oral ketotifen is used in Mexico and across Europe for the treatment of various allergic symptoms and disorders,[A231204] including urticaria, mastocytosis, and food allergy. |
| Indications and Usage |
Administered orally, ketotifen is indicated as an add-on medication in the chronic treatment of mild atopic asthma in children.[L32283] It is also available as an over-the-counter ophthalmic solution which is indicated for the temporary prevention of itching of the eye due to allergic conjunctivitis.[L32278] |
| Marketing Status |
approved |
| ATC Code |
R06AX17; S01GX08 |
| DrugBank ID |
DB00920
|
| KEGG ID |
D08105
|
| MeSH ID |
D007665
|
| PubChem ID |
3827
|
| TTD Drug ID |
D0YG7M
|
| NDC Product Code |
Not Available |
| UNII |
X49220T18G
|
| Synonyms |
Ketotifen | Ketotiphene | Ketotiphen | 4,9-Dihydro-4-(1-methyl-4-piperidylidene)-10H-benzo(4,5)-cyclohepta(1,2-b)thiophen-10-one | Ketotifene | Zaditen | Ketotifen Fumarate | Fumarate, Ketotifen |
|
| Chemical Information |
| Molecular Formula |
C19H19NOS |
| CAS Registry Number |
34580-13-7 |
| SMILES |
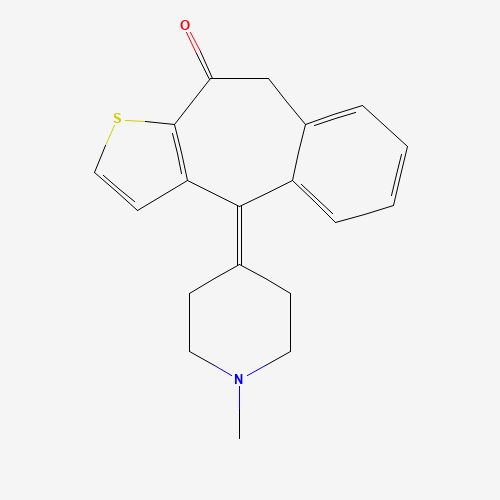
CN1CCC(=C2C3=C(C(=O)CC4=CC=CC=C42)SC=C3)CC1 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

