| Pharmaceutical Information |
| Drug Name |
Colestipol |
| Drug ID |
BADD_D00521 |
| Description |
Bile acid sequestrants like colestipol have been in use since the 1970s.[FDA Label, F4555, F4567, L6262] And even though such an agent may very well be useful in reducing elevated cholesterol levels and decreasing the risk for atherosclerotic vascular disease due to hypercholesterolemia, colestipol is still generally only employed as an adjunct therapy and the relatively physical nature of its pharmacological activity sometimes limits its usefulness.[FDA Label, F4555, F4567, L6262]
In particular, as colestipol's general mechanism of action ultimately results in the decreased absorption and enhanced secretion of bile acids and lipids in the feces, patients who take complicated medication regimens, experience constipation or biliary obstruction, etc. may not be good candidates for using the agent owing to its physical effects on the gut.[FDA Label, F4555, F4567, L6262]
Alternatively, colestipol predominantly elicits its activities within the gut environment because it undergoes little absorption and metabolism.[FDA Label, F4555, F4567, L6262] The resultant lack of systemic exposure consequently means the medication generally demonstrates very few adverse effects inside the body.[FDA Label, F4555, F4567, L6262] |
| Indications and Usage |
Colestipol is indicated as adjunctive therapy to diet for the reduction of elevated serum total and low-density lipoprotein cholesterol (LDL-C) in patients with primary hypercholesterolemia (a condition that features elevated LDL-C) who do not respond adequately to dietary changes .[FDA Label,L6115,F4555]
Therapy with lipid-altering agents like colestipol should be a component of multiple risk factor intervention in those individuals at significantly increased risk for atherosclerotic vascular disease due to hypercholesterolemia [FDA Label, L6115, F4555]. Treatment should begin and continue with dietary therapy [FDA Label, L6115, F4555]. In general, a minimum of six months of intensive dietary therapy and counseling should be carried out prior to initiation of drug therapy such as that with colestipol [FDA Label, F4555]. Shorter periods may be considered in patients with severe elevations of LDL-C or with definite coronary heart disease [FDA Label, F4555].
Although colestipol is effective in all types of hypercholesterolemia, some regional prescribing information note in particular that it is medically most appropriate in patients with Fredrickson's type II hyperlipoproteinemia [L6115]. Nevertheless, in patients with combined hypercholesterolemia and hypertriglyceridemia, although colestipol may be helpful in reducing elevated cholesterol, it is not formally indicated where hypertriglyceridemia is the abnormality of greatest concern [F4567]. |
| Marketing Status |
approved |
| ATC Code |
C10AC02 |
| DrugBank ID |
DB00375
|
| KEGG ID |
D07771
|
| MeSH ID |
D003084
|
| PubChem ID |
62816
|
| TTD Drug ID |
D0GJ8R
|
| NDC Product Code |
Not Available |
| UNII |
K50N755924
|
| Synonyms |
Colestipol | Colestipol Hydrochloride | Hydrochloride, Colestipol | Colestipol HCl | HCl, Colestipol | U-26,597 A | U 26,597 A | U26,597 A | Colestid |
|
| Chemical Information |
| Molecular Formula |
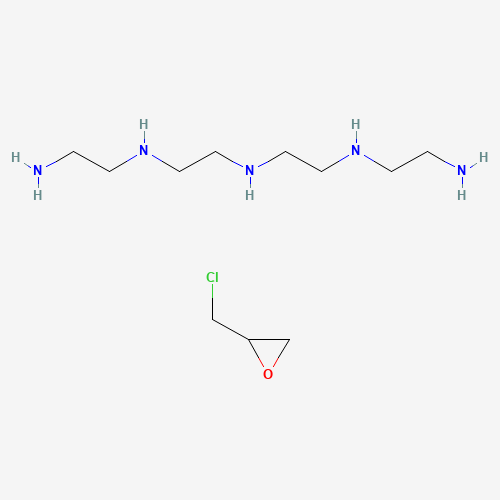
C11H28ClN5O |
| CAS Registry Number |
90366-83-9 |
| SMILES |
C1C(O1)CCl.C(CNCCNCCNCCN)N |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

