| Pharmaceutical Information |
| Drug Name |
Bisoprolol |
| Drug ID |
BADD_D00277 |
| Description |
Bisoprolol is a cardioselective β1-adrenergic blocking agent used to treat high blood pressure.[A180472,L7219] It is considered a potent drug with a long-half life that can be used once daily to reduce the need for multiple doses of antihypertensive drugs.[A180472] Bisoprolol is generally well tolerated, likely due to its β1-adrenergic receptor selectivity and is a useful alternative to non-selective β-blocker drugs in the treatment of hypertension such as [Carvedilol] and [Labetalol]. It may be used alone or in combination with other drugs to manage hypertension[L7219] and can be useful in patients with chronic obstructive pulmonary disease (COPD) due to its receptor selectivity.[A180562] |
| Indications and Usage |
Bisoprolol is indicated for the treatment of mild to moderate hypertension.[L7219] It may be used off-label to treat heart failure, atrial fibrillation, and angina pectoris.[A180460,A180463] |
| Marketing Status |
approved |
| ATC Code |
C07AB07 |
| DrugBank ID |
DB00612
|
| KEGG ID |
D02342
|
| MeSH ID |
D017298
|
| PubChem ID |
2405
|
| TTD Drug ID |
D0K3ZR
|
| NDC Product Code |
Not Available |
| UNII |
Y41JS2NL6U
|
| Synonyms |
Bisoprolol | Bisoprolol Fumarate (1:1) Salt, (+-)-Isomer | Bisoprolol Fumarate (2:1) Salt, (+-)-Isomer | Bisoprolol Hydrochloride | Hydrochloride, Bisoprolol | Bisoprolol Methanesulfonate Salt | Bisoprolol, (+-)-Isomer | Bisoprolol, Fumarate (1:1) Salt | Bisoprolol, Fumarate (2:1) Salt | CL-297939 | CL 297939 | CL297939 | Concor | EMD-33512 | EMD 33512 | EMD33512 | Bisoprolol Fumarate | Fumarate, Bisoprolol | Bisoprolol, (-)-Isomer |
|
| Chemical Information |
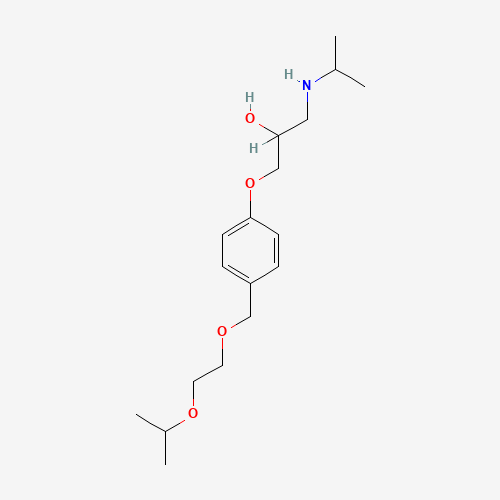
| Molecular Formula |
C18H31NO4 |
| CAS Registry Number |
66722-44-9 |
| SMILES |
CC(C)NCC(COC1=CC=C(C=C1)COCCOC(C)C)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

