| Pharmaceutical Information |
| Drug Name |
Netarsudil |
| Drug ID |
BADD_D02519 |
| Description |
A Rho kinase inhibitor with norepinephrine transport inhibitory activity that reduces production of aqueous
As of December 18, 2017 the FDA approved Aerie Pharmaceutical's Rhopressa (netarsudil ophthalmic solution) 0.02% for the indication of reducing elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Acting as both a rho kinase inhibitor and a norepinephrine transport inhibitor, Netarsudil is a novel glaucoma medication in that it specifically targets the conventional trabecular pathway of aqueous humour outflow to act as an inhibitor to the rho kinase and norepinephrine transporters found there as opposed to affecting protaglandin F2-alpha analog like mechanisms in the unconventional uveoscleral pathway that many other glaucoma medications demonstrate. |
| Indications and Usage |
Netarsudil is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension [FDA Label]. |
| Marketing Status |
approved |
| ATC Code |
S01EX05 |
| DrugBank ID |
DB13931
|
| KEGG ID |
D11030
|
| MeSH ID |
C000603944
|
| PubChem ID |
66599893
|
| TTD Drug ID |
D04WYX
|
| NDC Product Code |
70727-497 |
| UNII |
W6I5QDT7QI
|
| Synonyms |
netarsudil | AR-13324 |
|
| Chemical Information |
| Molecular Formula |
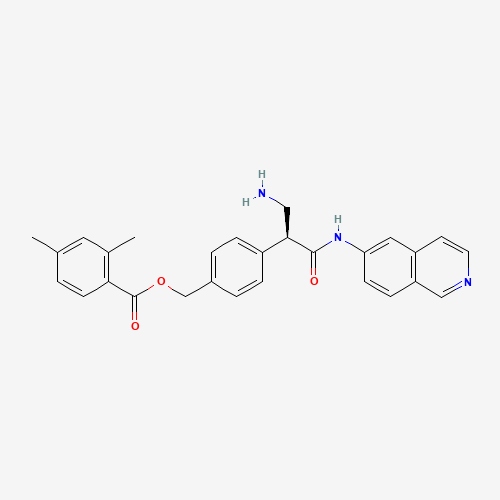
C28H27N3O3 |
| CAS Registry Number |
1254032-66-0 |
| SMILES |
CC1=CC(=C(C=C1)C(=O)OCC2=CC=C(C=C2)C(CN)C(=O)NC3=CC4=C(C=C3)C=NC=C4)C |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

