| Pharmaceutical Information |
| Drug Name |
Esketamine |
| Drug ID |
BADD_D02488 |
| Description |
Major depressive disorder (MDD) is a significant cause of disability worldwide and the most common illness preceding suicide [L5596], [A175462]. On March 5, 2019, the nasal spray drug, _esketamine_, also known as _Spravato_ (by Janssen Pharmaceuticals), was approved by the FDA for treatment-resistant major depression.
Esketamine is the s-enantiomer of [Ketamine]. Ketamine is a mixture of two enantiomers (mirror image molecules). This is the first time that the FDA has approved esketamine for any use. The FDA approved ketamine (Ketalar) in 1970 [L5593].
Esketamine may prove to be a promising treatment for patients diagnosed with major depressive disorder who have not experienced an improvement in symptoms despite treatment with various medications and therapies. The intranasal route of administration for this drug allows for easy administration and a fast onset of action, which sets it apart from many other antidepressant agents that may take several weeks to take effect. |
| Indications and Usage |
This drug is indicated in conjunction with an oral antidepressant for the treatment of treatment-resistant depression (TRD) in adults [FDA label].
Note: Esketamine is not approved as an anesthetic agent. The safety and effectiveness of esketamine as an anesthetic agent have not been established to this date [FDA label]. |
| Marketing Status |
approved; investigational |
| ATC Code |
N01AX14; N06AX27 |
| DrugBank ID |
DB11823
|
| KEGG ID |
D07283
|
| MeSH ID |
C000629870
|
| PubChem ID |
182137
|
| TTD Drug ID |
D0TP5H
|
| NDC Product Code |
65267-117 |
| UNII |
50LFG02TXD
|
| Synonyms |
Esketamine | Kataved | S-Ketamine | (S)-2-(o-chlorophenyl)-2-(methylamino)cyclohexanone | L-Ketamine | (-)-Ketamine | Spravato |
|
| Chemical Information |
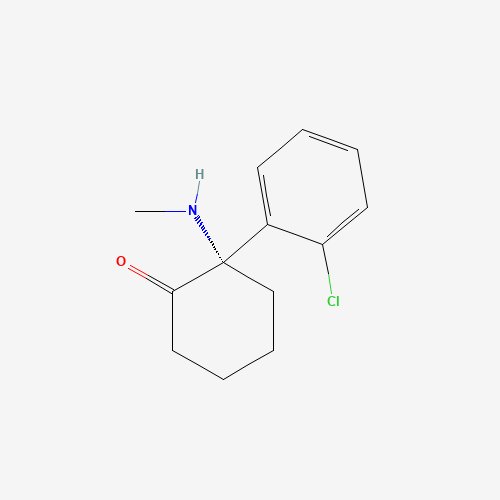
| Molecular Formula |
C13H16ClNO |
| CAS Registry Number |
33643-46-8 |
| SMILES |
CNC1(CCCCC1=O)C2=CC=CC=C2Cl |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

