| Pharmaceutical Information |
| Drug Name |
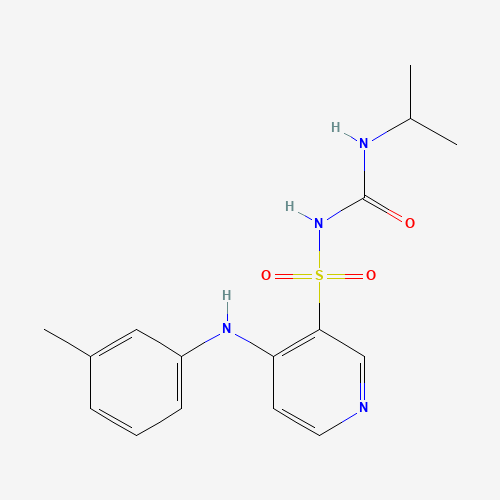
Torasemide |
| Drug ID |
BADD_D02413 |
| Description |
Torasemide is a high-ceiling loop diuretic.[A174463] Structurally, it is a pyridine-sulfonylurea used as an antihypertensive agent.[A319] Torasemide was first approved for clinical use by the FDA in 1993.[L5257] |
| Indications and Usage |
Torasemide is indicated for the treatment of edema associated with congestive heart failure, renal or hepatic diseases. From this condition, it has been observed that torasemide is very effective in cases of kidney failure.[FDA label]
Edema is considered when swelling is observed due to the trap of fluid in the body tissue. It is mainly located in feet, ankles and legs but it can also be extended to other parts such as face, hands and abdomen or even the whole body.[L5251]
As well, torasemide is approved to be used as an antihypertensive agent either alone or in combination with other antihypertensives.[FDA label]
Hypertension is defined by the presence of high blood pressure. This is caused by an increase in the amount of blood pumped which in order produces the narrowing of the arteries.[L5254] |
| Marketing Status |
Not Available |
| ATC Code |
C03CA04 |
| DrugBank ID |
DB00214
|
| KEGG ID |
D00382
|
| MeSH ID |
D000077786
|
| PubChem ID |
41781
|
| TTD Drug ID |
D0J9XZ
|
| NDC Product Code |
10920-584 |
| Synonyms |
Torsemide | Torasemide | 1-Isopropyl-3-((4-m-toluidino-3-pyridyl)sulfonyl)urea | 1-Isopropyl-3-((4-(3-methylphenylamino)pyridine)-3-sulfonyl)urea | Demadex |
|
| Chemical Information |
| Molecular Formula |
C16H20N4O3S |
| CAS Registry Number |
56211-40-6 |
| SMILES |
CC1=CC(=CC=C1)NC2=C(C=NC=C2)S(=O)(=O)NC(=O)NC(C)C |
| Chemical Structure |
|
|
| ADR Related Proteins Induced by Drug |
| ADR Term |
Protein Name |
UniProt AC |
TTD Target ID |
PMID |
| Not Available | Not Available | Not Available | Not Available | Not Available |
|
| ADRs Induced by Drug |
|
|

