| Pharmaceutical Information |
| Drug Name |
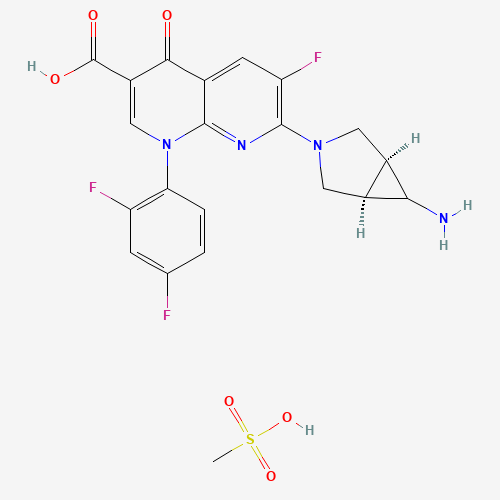
Trovafloxacin mesylate |
| Drug ID |
BADD_D02308 |
| Description |
Trovafloxacin is a broad spectrum antibiotic that has been commonly marketed under the brand name Trovan by Pfizer. It exerts its antibacterial activity by inhibiting the uncoiling of supercoiled DNA in various bacteria by blocking the activity of DNA gyrase and topoisomerase IV. It was shown to be more effective against Gram-positive bacteria than Gram-negative bacteria when compared to previous fluoroquinolones. Due to its hepatotoxic potential, trovafloxacin was withdrawn from the market. |
| Indications and Usage |
For treatment of infections caused by susceptible strains of the designated microorganisms in uncomplicated urethral gonorrhea in males and endocervical and rectal gonorrhea in females caused by Neisseria gonorrhoeae as well as non gonoccocal urethritis and cervicitis due to Chlamydia trachomatis. |
| Marketing Status |
approved; investigational; withdrawn |
| ATC Code |
J01MA13 |
| DrugBank ID |
DB00685
|
| KEGG ID |
D02123
|
| MeSH ID |
C080163
|
| PubChem ID |
62960
|
| TTD Drug ID |
D03WPA
|
| NDC Product Code |
Not Available |
| UNII |
0P1LKO80WN
|
| Synonyms |
trovafloxacin | CP 99219 | CP-99,219 | CP-99219 | CP 99,219 |
|
| Chemical Information |
| Molecular Formula |
C21H19F3N4O6S |
| CAS Registry Number |
147059-75-4 |
| SMILES |
CS(=O)(=O)O.C1C2C(C2N)CN1C3=C(C=C4C(=O)C(=CN(C4=N3)C5=C(C=C(C=C5)F)F)C(=O)O)F |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

