| Pharmaceutical Information |
| Drug Name |
Reserpine |
| Drug ID |
BADD_D01926 |
| Description |
An alkaloid found in the roots of Rauwolfia serpentina and R. vomitoria. Reserpine inhibits the uptake of norepinephrine into storage vesicles resulting in depletion of catecholamines and serotonin from central and peripheral axon terminals. It has been used as an antihypertensive and an antipsychotic as well as a research tool, but its adverse effects limit its clinical use. |
| Indications and Usage |
For the treatment of hypertension |
| Marketing Status |
approved; investigational |
| ATC Code |
C02AA02 |
| DrugBank ID |
DB00206
|
| KEGG ID |
D00197
|
| MeSH ID |
D012110
|
| PubChem ID |
5770
|
| TTD Drug ID |
D0J4JM
|
| NDC Product Code |
49452-6217; 71052-422; 17359-1000 |
| UNII |
8B1QWR724A
|
| Synonyms |
Reserpine | Rausedil | Rausedyl | Serpasil | Raunervil | V-Serp | V Serp | Raupasil | Serpivite |
|
| Chemical Information |
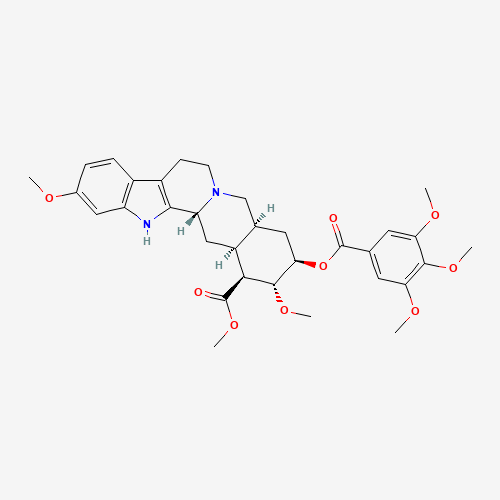
| Molecular Formula |
C33H40N2O9 |
| CAS Registry Number |
50-55-5 |
| SMILES |
COC1C(CC2CN3CCC4=C(C3CC2C1C(=O)OC)NC5=C4C=CC(=C5)OC)OC(=O)C6=CC(=C(C(=C6)OC)OC)O
C |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

