| Pharmaceutical Information |
| Drug Name |
Pralidoxime |
| Drug ID |
BADD_D01815 |
| Description |
Pralidoxime is an antidote to organophosphate pesticides and chemicals. Organophosphates bind to the esteratic site of acetylcholinesterase, which results initially in reversible inactivation of the enzyme. If given within 24 hours,after organophosphate exposure, pralidoxime reactivates the enzyme cholinesterase by cleaving the phosphate-ester bond formed between the organophosphate and acetylcholinesterase. |
| Indications and Usage |
For the treatment of poisoning due to those pesticides and chemicals of the organophosphate class which have anticholinesterase activity and in the control of overdosage by anticholinesterase drugs used in the treatment of myasthenia gravis. |
| Marketing Status |
approved; vet_approved |
| ATC Code |
V03AB04 |
| DrugBank ID |
DB00733
|
| KEGG ID |
D00469
|
| MeSH ID |
C028797
|
| PubChem ID |
4884
|
| TTD Drug ID |
D0X7NU
|
| NDC Product Code |
Not Available |
| UNII |
P7MU9UTP52
|
| Synonyms |
pralidoxime | 2-PAM | 2-hydroxyiminomethyl-1-methylpyridinium | 1-methylpyridinium-2-aldoxime ion | pralidoxime bromide | 2-Pam bromide | pralidoxime chloride | 2-formyl-1-methylpyridinium chloride oxime | pyridine-2-aldoxime methochloride | 2-PAM chloride | pyridine-2-aldoxime methachloride | pralidoxime fumarate (1:1) | pralidoxime iodide | pyridine-2-aldoxime methiodide | pralidoxime lactate (1:1) | pralidoxime mesylate | 2-hydroxyiminomethylpyridinium methylmethanesulfonate | pralidoxime methyl sulfate | N-methylpyridinium 2-aldoxime methylsulfate | pralidoxime nitrate (1:1) | pralidoxime sulfate (1:1) | pralidoxime trichloroacetate | pralidoxime, 14C-labeled | Protopam | Protopam Chloride | Contrathion |
|
| Chemical Information |
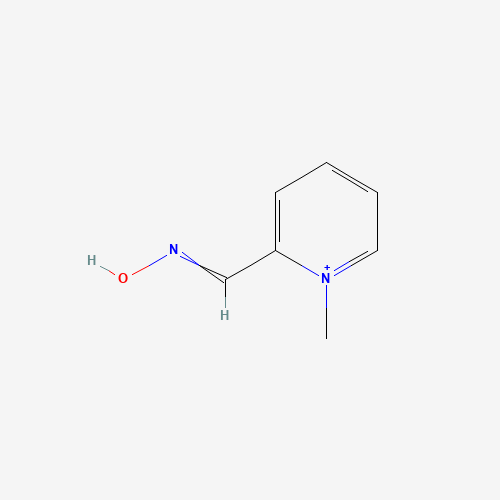
| Molecular Formula |
C7H9N2O+ |
| CAS Registry Number |
6735-59-7 |
| SMILES |
C[N+]1=CC=CC=C1C=NO |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

