| Pharmaceutical Information |
| Drug Name |
Phentermine |
| Drug ID |
BADD_D01756 |
| Description |
Phentermine is a sympathomimetic amine anorectic agent and it was introduced in 1959 as part of an anti-obesity combination drug.[A174361, A174364] It is chemically related to amphetamine and it is commonly referred to as an atypical amphetamine.[A174370] Phentermine has not been reported an addictive potential which allows this agent to be classified under the Schedule IV drugs (low abuse potential).[A174367]
Phentermine was FDA approved for short-term weight management in 1959 and it became widely used in 1960. This initial product, formed by the combination of phentermine with [fenfluramine] and [dexfenfluramine] was discontinued after finding several reports of abnormal valves in nearly 30% of the consumers.[A174376, T403] Later on, phentermine was approved alone and in combination with topiramate in 2012 as a new alternative that required lower doses of phentermine to obtain the desired effect.[A174373] |
| Indications and Usage |
Phentermine is indicated, alone or in combination with topiramate, as a short-term adjunct, not pass a few weeks, in a regimen of weight reduction based on exercise, behavioral modifications and caloric restriction in the management of exogenous obesity for patients with an initial body mass index (BMI) greater than 30 kg/m2 or greater than 27 kg/m2 in presence of other risk factors such as controller hypertension, diabetes or hyperlipidemia.[FDA label]
Exogenous obesity is considered when the overweight is caused by consuming more food than the person activity level warrants. This condition commonly causes an increase in fat storage. It is an epidemic condition in the United States where over two-thirds of adults are overweight or obese and one in three Americans is obese. In the world, the incidence of obesity has nearly doubled.[A174391] |
| Marketing Status |
approved; illicit |
| ATC Code |
A08AA01 |
| DrugBank ID |
DB00191
|
| KEGG ID |
D05458
|
| MeSH ID |
D010645
|
| PubChem ID |
4771
|
| TTD Drug ID |
D0U0RZ
|
| NDC Product Code |
Not Available |
| UNII |
C045TQL4WP
|
| Synonyms |
Phentermine | Duromine | Phentermine Hydrochloride | Hydrochloride, Phentermine | Adipex-P | Adipex P | AdipexP | Ionamine |
|
| Chemical Information |
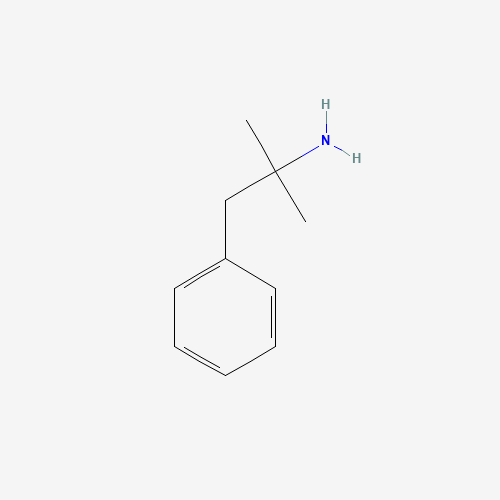
| Molecular Formula |
C10H15N |
| CAS Registry Number |
122-09-8 |
| SMILES |
CC(C)(CC1=CC=CC=C1)N |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

