| Pharmaceutical Information |
| Drug Name |
Pentobarbital sodium |
| Drug ID |
BADD_D01727 |
| Description |
A short-acting barbiturate that is effective as a sedative and hypnotic (but not as an anti-anxiety) agent and is usually given orally. It is prescribed more frequently for sleep induction than for sedation but, like similar agents, may lose its effectiveness by the second week of continued administration. (From AMA Drug Evaluations Annual, 1994, p236) |
| Indications and Usage |
For the short-term treatment of insomnia. |
| Marketing Status |
approved; investigational; vet_approved |
| ATC Code |
N05CA01 |
| DrugBank ID |
DB00312
|
| KEGG ID |
D00500
|
| MeSH ID |
D010424
|
| PubChem ID |
23676152
|
| TTD Drug ID |
D0F0YZ
|
| NDC Product Code |
72735-010; 25021-676; 24201-010; 0792-0045; 76478-501; 0792-0044; 17478-181; 53834-601; 65392-0227 |
| UNII |
NJJ0475N0S
|
| Synonyms |
Pentobarbital | Pentobarbitone | Mebubarbital | Etaminal | Ethaminal | Sagatal | Nembutal | Pentobarbital Sodium | Pentobarbital, Monosodium Salt | Monosodium Salt Pentobarbital | Diabutal | Mebumal |
|
| Chemical Information |
| Molecular Formula |
C11H17N2NaO3 |
| CAS Registry Number |
57-33-0 |
| SMILES |
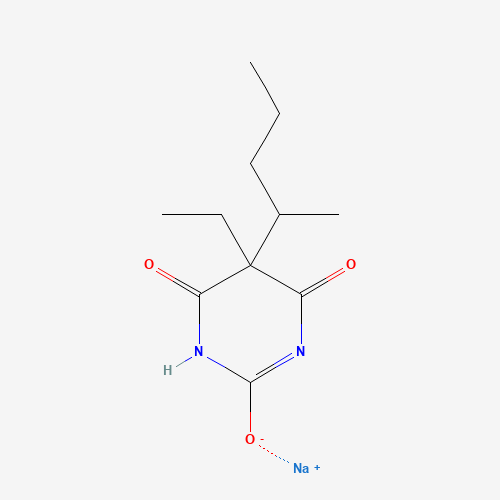
CCCC(C)C1(C(=O)NC(=NC1=O)[O-])CC.[Na+] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Liver injury | 12.01.17.012; 09.01.07.022 | - | - | Not Available |
|
|
|

