| Pharmaceutical Information |
| Drug Name |
Oseltamivir |
| Drug ID |
BADD_D01627 |
| Description |
Oseltamivir (marketed as the product TamifluⓇ), is an antiviral neuraminidase inhibitor used for the treatment and prophylaxis of infection with influenza viruses A (including pandemic H1N1) and B. Oseltamivir exerts its antiviral activity by inhibiting the activity of the viral neuraminidase enzyme found on the surface of the virus, which prevents budding from the host cell, viral replication, and infectivity.
The clinical benefit of use of oseltamivir is greatest when administered within 48 hours of the onset of influenza symptoms since effectiveness decreases significantly after that point in time; there is generally no benefit in use beyond 48 hours for healthy, low-risk individuals as influenza is a self-limiting illness.[A180574, L7267, A180580] However, antiviral treatment might be beneficial when initiated after 48 hours for patients with severe, complicated or progressive illness or for hospitalized patients.[A180583] According to the CDC, data from clinical trials and observational studies have demonstrated that early antiviral treatment can shorten the duration of fever and illness symptoms, and may reduce the risk of some complications (including pneumonia and respiratory failure). They recommend the use of oseltamivir in people with a higher risk of developing complications including children younger than 2 years, people over 65 years, people with some chronic conditions or immunosuppression, pregnant women, residents of long term care facilities, and indigenous communities for example.[L7264]
The benefits of oseltamivir use are controversial; a 2014 Cochrane Review of the evidence found that oseltamivir treatment had limited benefit. The authors concluded that oseltamivir use in healthy adults had small, non-specific effects on symptoms (where the time to first alleviation of symptoms was only reduced from 7 to 6.3 days), it had no effect on hospitalizations, and that there was no evidence for any reductions in complications of influenza such as pneumonia.[A179962, A179965, A179968] Due to the risk of adverse effects such as nausea, vomiting, psychiatric effects and renal adverse events in adults and vomiting in children, the harms are generally considered to outweigh the small clinical benefit of use of oseltamivir.
Notably, in 2017, the World Health Organization downgraded oseltamivir from its essential medicines list from a "core" drug to a "complementary" drug, due to limited cost-effectiveness.[A179086] Yearly vaccination with the influenza vaccine is still considered the best preventative measure. |
| Indications and Usage |
According to FDA prescribing information, oseltamivir is indicated for the treatment of acute, uncomplicated illness due to influenza A and B infection in patients 2 weeks of age and older who have been symptomatic for no more than 48 hours [F3094]. In particular, this agent is indicated in adults and children including full-term neonates who present with symptoms typical of influenza when influenza virus is circulating in the community. Efficacy has been demonstrated when treatment is initiated within two days of the first onset of symptoms.[F3097]
Oseltamivir is also indicated for the prophylaxis of influenza in patients one year and older [F3094]. Specifically, post-exposure prevention in individuals one year of age or older following contact with a clinically diagnosed influenza case when influenza virus is circulating in the community qualifies for such prophylactic therapy. Oseltamivir would only be indicated for post-exposure prevention of influenza in infants less than 1 year of age during a pandemic influenza outbreak. [F3097] |
| Marketing Status |
approved |
| ATC Code |
J05AH02 |
| DrugBank ID |
DB00198
|
| KEGG ID |
D08306
|
| MeSH ID |
D053139
|
| PubChem ID |
65028
|
| TTD Drug ID |
D0O5NK
|
| NDC Product Code |
46708-413; 60219-1265; 62332-415; 69238-1264; 62332-413; 60219-1264; 60219-1266; 62332-414; 46708-414; 46708-415; 42291-664; 69238-1265; 69238-1266; 42291-666 |
| UNII |
20O93L6F9H
|
| Synonyms |
Oseltamivir | GS 4104 | GS4104 | GS-4104 | Tamiflu | GS 4071 | GS4071 | GS-4071 |
|
| Chemical Information |
| Molecular Formula |
C16H28N2O4 |
| CAS Registry Number |
196618-13-0 |
| SMILES |
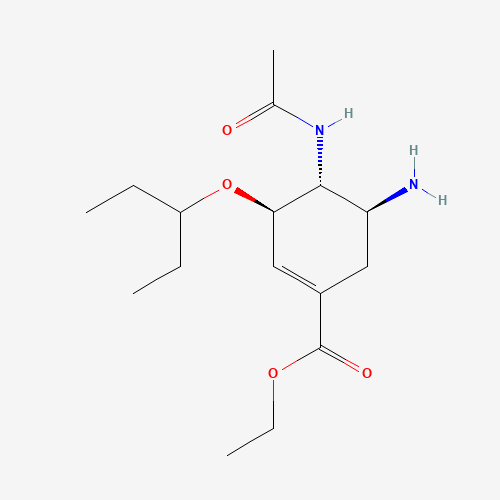
CCC(CC)OC1C=C(CC(C1NC(=O)C)N)C(=O)OCC |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

