| Pharmaceutical Information |
| Drug Name |
Melphalan hydrochloride |
| Drug ID |
BADD_D01377 |
| Description |
An alkylating nitrogen mustard that is used as an antineoplastic in the form of the levo isomer - melphalan, the racemic mixture - merphalan, and the dextro isomer - medphalan; toxic to bone marrow, but little vesicant action; potential carcinogen. |
| Indications and Usage |
For the palliative treatment of multiple myeloma and for the palliation of non-resectable epithelial carcinoma of the ovary. Has also been used alone or as part of various chemotherapeutic regimens as an adjunct to surgery in the treatment of breast cancer, alone or in combination regimens for palliative treatment of locally recurrent or unresectable in-transit metastatic melanoma of the extremities, as well as for the treatment of amyloidosis with prednisone. |
| Marketing Status |
approved |
| ATC Code |
L01AA03 |
| DrugBank ID |
DB01042
|
| KEGG ID |
D08173
|
| MeSH ID |
D008558
|
| PubChem ID |
9927978
|
| TTD Drug ID |
D00FGO
|
| NDC Product Code |
59651-684; 63552-076; 17337-0304; 68554-0087; 76055-0011; 43598-392; 67457-215; 45963-686; 70860-214; 71288-112; 71288-132; 76339-111; 54288-109; 70700-278; 62207-978; 43598-027; 72266-128; 46439-8735; 63323-760; 67457-195; 68083-259; 59981-016; 72611-779; 82920-020 |
| UNII |
1VXP4V453T
|
| Synonyms |
Melphalan | L-PAM | Phenylalanine Mustard | Mustard, Phenylalanine | 4-(Bis(2-chloroethyl)amino)phenylalanine | Medphalan | Sarkolysin | Sarcolysine | Merphalan | Alkeran |
|
| Chemical Information |
| Molecular Formula |
C13H19Cl3N2O2 |
| CAS Registry Number |
3223-07-2 |
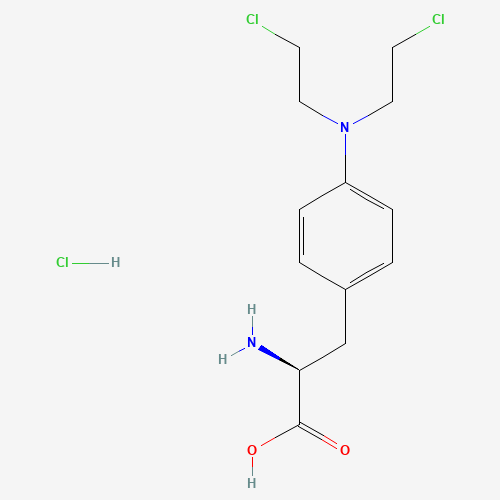
| SMILES |
C1=CC(=CC=C1CC(C(=O)O)N)N(CCCl)CCCl.Cl |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

