| Pharmaceutical Information |
| Drug Name |
Isocarboxazid |
| Drug ID |
BADD_D01202 |
| Description |
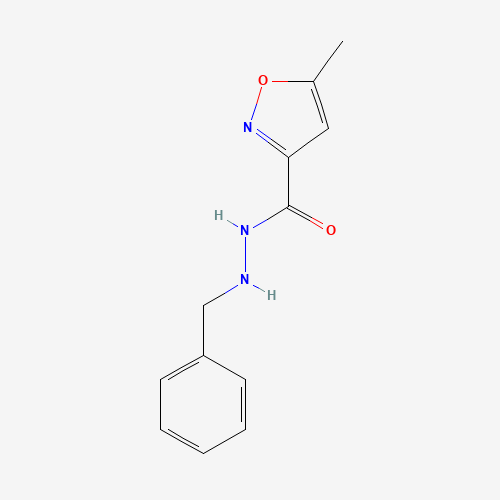
Isocarboxazid has the formula 1-benzyl-2-(5-methyl-3-isoxazolylcarbonyl)hydrazine-isocarboxazid. It is a monoamine oxidase inhibitor.[A31930] It is used in the treatment of major depression, dysthymic disorder, atypical disorder, panic disorder and the phobic disorders.[T115] It was first introduced by Roche pharmaceuticals, further developed by Validus pharms Inc and first FDA approved as a prescription drug on July 1st, 1959. |
| Indications and Usage |
Isocarboxazid is indicated for the treatment of the enduring and debilitating symptoms of depression that have not responded to other antidepressant drugs.[L1372] Depression is a common but serious mood disorder. The patient will present changes in its feelings, thoughts, and ability to handle everyday activities. For a mood disorder to be considered as depression, the symptoms should be present for at least two weeks.[L1375] |
| Marketing Status |
approved |
| ATC Code |
N06AF01 |
| DrugBank ID |
DB01247
|
| KEGG ID |
D02580
|
| MeSH ID |
D007520
|
| PubChem ID |
3759
|
| TTD Drug ID |
D0I2VK
|
| NDC Product Code |
55679-115; 30698-032 |
| UNII |
34237V843T
|
| Synonyms |
Isocarboxazid |
|
| Chemical Information |
| Molecular Formula |
C12H13N3O2 |
| CAS Registry Number |
59-63-2 |
| SMILES |
CC1=CC(=NO1)C(=O)NNCC2=CC=CC=C2 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

