| Pharmaceutical Information |
| Drug Name |
Histrelin |
| Drug ID |
BADD_D01073 |
| Description |
Histrelin is a gonadotropin releasing hormone (GnRH) agonist that acts as a potent inhibitor of gonadotropin when administered as an implant that delivers continuous therapeutic doses. Following an initial stimulatory phase with increased circulating levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), leading to a transient increase in concentration of gonadal steroids (testosterone and dihydrotestosterone in males), continuous administration of histrelin acetate results in decreased levels of LH and FSH due to a reversible down-regulation of the GnRH receptors in the pituitary gland and desensitization of the pituitary gonadotropes.
As the product Supprelin LA (FDA), histrelin is indicated for the treatment of children with central precocious puberty (CPP). As the product Vantas (FDA), histrelin is indicated for the palliative treatment of advanced prostate cancer. |
| Indications and Usage |
As the product Supprelin LA (FDA), histrelin is indicated for the treatment of children with central precocious puberty (CPP). As the product Vantas (FDA), histrelin is indicated for the palliative treatment of advanced prostate cancer. |
| Marketing Status |
approved |
| ATC Code |
L02AE05 |
| DrugBank ID |
DB06788
|
| KEGG ID |
D02369
|
| MeSH ID |
C029256
|
| PubChem ID |
25077993
|
| TTD Drug ID |
D0O7DG
|
| NDC Product Code |
Not Available |
| UNII |
H50H3S3W74
|
| Synonyms |
histrelin | 6-His(imBzl)-9-N-Et-ProNH2-10-des-GlyNH2-LHRH | GnRH, His(imBzl)(6)-N-Et-ProNH2(9)- | LHRH, His(imBzl)(6)-N-Et-ProNH2(9)- | LHRH, histidyl(imBzl)(6)-N-ethylprolinamide(9)-des-glycinamide(10)- | imbzl-His(6), Pro(9)-NET-GNRH | ((im bzl)-D-His(6), Pro(9)-NEt)LHRH | IBHPE-LHRH | Supprelin |
|
| Chemical Information |
| Molecular Formula |
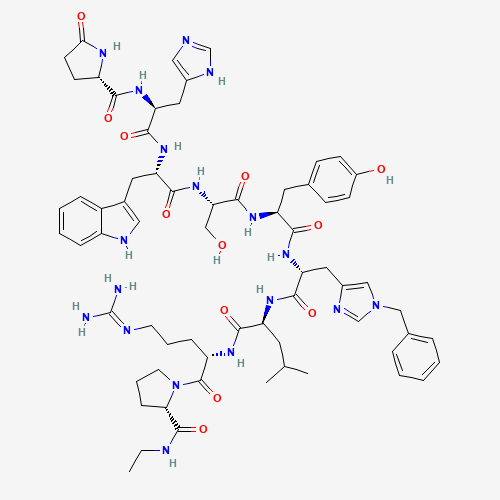
C66H86N18O12 |
| CAS Registry Number |
76712-82-8 |
| SMILES |
CCNC(=O)C1CCCN1C(=O)C(CCCN=C(N)N)NC(=O)C(CC(C)C)NC(=O)C(CC2=CN(C=N2)CC3=CC=CC=C3
)NC(=O)C(CC4=CC=C(C=C4)O)NC(=O)C(CO)NC(=O)C(CC5=CNC6=CC=CC=C65)NC(=O)C(CC7=CN=CN
7)NC(=O)C8CCC(=O)N8 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

