| Pharmaceutical Information |
| Drug Name |
Elvitegravir |
| Drug ID |
BADD_D00762 |
| Description |
Elvitegravir is a human immunodeficiency virus type 1 (HIV-1) integrase strand transfer inhibitor (INSTI) used for the treatment of HIV-1 infection in antiretroviral treatment-experienced adults. Because integrase is necessary for viral replication, inhibition prevents the integration of HIV-1 DNA into the host genome and thereby blocks the formation of the HIV-1 provirus and resulting propagation of the viral infection. Although available as a single dose tablet, elvitegravir must be used in combination with an HIV protease inhibitor coadministered with ritonavir and another antiretroviral drug.
Elvitegravir was first licensed from Japan Tobacco in 2008 and developed by Gilead Sciences. It was FDA approved on August 27, 2012. On September 24, 2014, the FDA approved the single pill form of elvitegravir. |
| Indications and Usage |
Elvitegravir in combination with an HIV protease inhibitor coadministered with ritonavir and with other antiretroviral drug(s) is indicated for the treatment of HIV-1 infection in antiretroviral treatment-experienced adults. |
| Marketing Status |
approved |
| ATC Code |
J05AJ02 |
| DrugBank ID |
DB09101
|
| KEGG ID |
D06677
|
| MeSH ID |
C509700
|
| PubChem ID |
5277135
|
| TTD Drug ID |
D0QD1G
|
| NDC Product Code |
69766-027; 65015-839; 66721-600; 48087-0107; 69037-0002; 65015-866 |
| UNII |
4GDQ854U53
|
| Synonyms |
elvitegravir | Vitekta | GS-9137 | GS9137 | GS 9137 | JTK-303 | JTK303 | JTK 303 |
|
| Chemical Information |
| Molecular Formula |
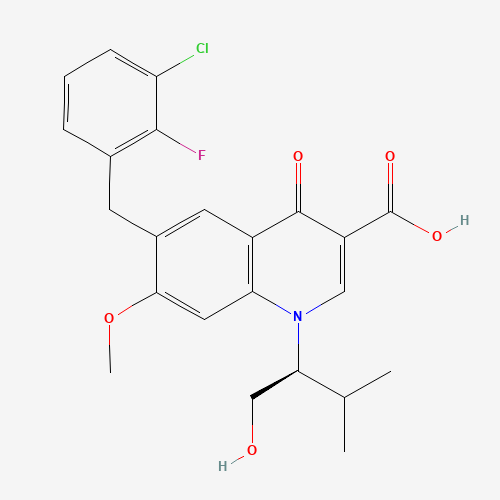
C23H23ClFNO5 |
| CAS Registry Number |
697761-98-1 |
| SMILES |
CC(C)C(CO)N1C=C(C(=O)C2=C1C=C(C(=C2)CC3=C(C(=CC=C3)Cl)F)OC)C(=O)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

