| Pharmaceutical Information |
| Drug Name |
Donepezil |
| Drug ID |
BADD_D00706 |
| Description |
In 2016, the global burden of dementia was estimated to be 43.8 million, demonstrating a significant increase from a global prevalence of 20.2 million in 1990.[A182411] Donepezil, also known as Aricept, is a piperidine derivative acetylcholinesterase inhibitor used in the management of the dementia of Alzheimer's Disease, and in some cases, is used to manage other types of dementia.
Donepezil was first approved by the FDA in 1996, and its extended-release form was approved in combination with [Memantine] in 2014 to manage moderate and severe forms of Alzheimer's dementia.[L7916,L7937] Though it does not alter the progression of Alzheimer's disease, donepezil is effective in managing the symptoms of its associated dementia.[T668] |
| Indications and Usage |
Donepezil is indicated for the management of mild to moderate Alzheimer’s Disease at doses of 5 mg or 10 mg.[L7916] It is also indicated for the management of moderate to severe Alzheimer’s Disease in a higher dose of 10 mg or 23 mg administered once daily.[L7916] Off-label uses include the management of vascular dementia, Parkinson's Disease-associated dementia, and Lewy body dementia, among others.[A182333,T668] When combined with memantine, the extended-release form of donepezil is indicated to treat the symptoms of moderate to severe dementia.[L7937] |
| Marketing Status |
approved |
| ATC Code |
N06DA02 |
| DrugBank ID |
DB00843
|
| KEGG ID |
D07869
|
| MeSH ID |
D000077265
|
| PubChem ID |
3152
|
| TTD Drug ID |
D0NS6H
|
| NDC Product Code |
65085-0046 |
| UNII |
8SSC91326P
|
| Synonyms |
Donepezil | Eranz | Donepezilium Oxalate Trihydrate | E 2020 | E2020 | E-2020 | Donepezil Hydrochloride | 1-Benzyl-4-((5,6-dimethoxy-1-indanon)-2-yl)methylpiperidine hydrochloride | Aricept |
|
| Chemical Information |
| Molecular Formula |
C24H29NO3 |
| CAS Registry Number |
120014-06-4 |
| SMILES |
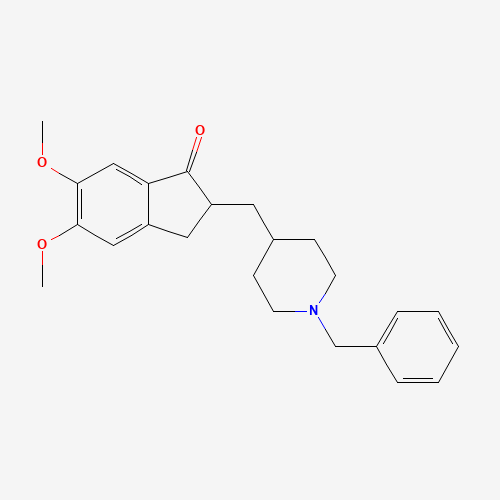
COC1=C(C=C2C(=C1)CC(C2=O)CC3CCN(CC3)CC4=CC=CC=C4)OC |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

