| Pharmaceutical Information |
| Drug Name |
Butabarbital |
| Drug ID |
BADD_D00319 |
| Description |
Butabarbital, or Butisol, is a fast onset barbiturate with short duration of action compared to other barbiturates.[A201977,L13613] This makes butabarbital a useful drug for treating severe insomnia and pre-operative anxiety.[A201977,L13613] Butabarbital is less commonly used in recent years, as more patients are typically prescribed benzodiazepines.[A19735] Its short duration of action gives butabarbital a high abuse potential, comparable to [secobarbital].[A201977,A201980]
Butabarbital was granted FDA approval on 5 June 1939.[L13613] |
| Indications and Usage |
Butabarbital is indicated for use as a sedative or hypnotic.[L13613] Butabarbital should not be used to treat insomnia for longer than 2 weeks.[L13613] |
| Marketing Status |
approved; illicit |
| ATC Code |
Not Available |
| DrugBank ID |
DB00237
|
| KEGG ID |
D03180
|
| MeSH ID |
C084833
|
| PubChem ID |
2479
|
| TTD Drug ID |
D0A4JK
|
| NDC Product Code |
61960-2400 |
| UNII |
P0078O25A9
|
| Synonyms |
secbutabarbital | butabarbital | secumalum | butabarbitone | secbutobarbitone | Butisol Sodium | Butisol | Sarisol | secbutabarbital sodium | butabarbital sodium | 2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-ethyl-5-(1-methylpropyl)-, monosodium salt |
|
| Chemical Information |
| Molecular Formula |
C10H16N2O3 |
| CAS Registry Number |
125-40-6 |
| SMILES |
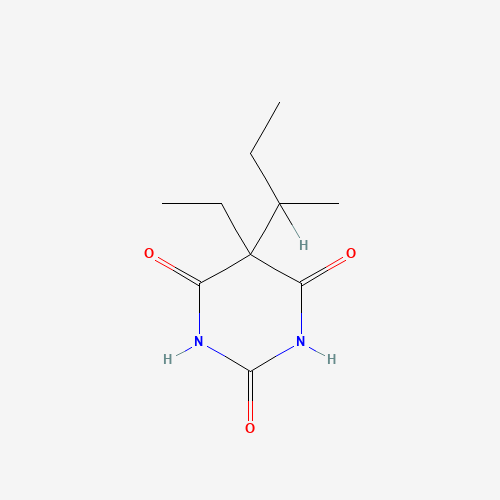
CCC(C)C1(C(=O)NC(=O)NC1=O)CC |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Syncope | 02.11.04.015; 24.06.02.012; 17.02.04.008 | - | - | | | Tension | 19.06.02.005 | - | - | Not Available | | Vomiting | 07.01.07.003 | - | - | | | Liver injury | 12.01.17.012; 09.01.07.022 | - | - | Not Available |
|
|
|

