| Pharmaceutical Information |
| Drug Name |
Aminoglutethimide |
| Drug ID |
BADD_D00107 |
| Description |
An aromatase inhibitor that produces a state of "medical" adrenalectomy by blocking the production of adrenal steroids. It also blocks the conversion of androgens to estrogens. Aminoglutethimide has been used in the treatment of advanced breast and prostate cancer. It was formerly used for its weak anticonvulsant properties. (From Martindale, The Extra Pharmacopoeia, 30th ed, p454) |
| Indications and Usage |
For the suppression of adrenal function in selected patients with Cushing's syndrome, malignant neoplasm of the female breast, and carcinoma in situ of the breast. |
| Marketing Status |
approved; investigational |
| ATC Code |
L02BG01 |
| DrugBank ID |
DB00357
|
| KEGG ID |
D00574
|
| MeSH ID |
D000616
|
| PubChem ID |
2145
|
| TTD Drug ID |
D0M6DO
|
| NDC Product Code |
Not Available |
| UNII |
0O54ZQ14I9
|
| Synonyms |
Aminoglutethimide | Cytadren | Orimeten |
|
| Chemical Information |
| Molecular Formula |
C13H16N2O2 |
| CAS Registry Number |
125-84-8 |
| SMILES |
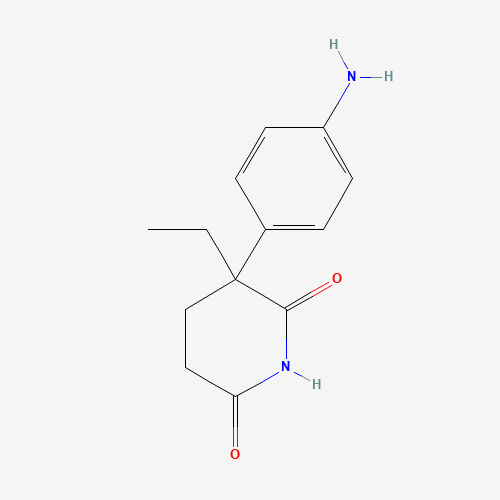
CCC1(CCC(=O)NC1=O)C2=CC=C(C=C2)N |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Skin abrasion | 12.01.06.010; 23.03.11.018 | - | - | Not Available | | Liver injury | 12.01.17.012; 09.01.07.022 | - | - | Not Available | | Hypersensitivity pneumonitis | 22.01.01.027; 10.01.03.056 | - | - | Not Available |
|
|
|

