| Pharmaceutical Information |
| Drug Name |
Tiotropium |
| Drug ID |
BADD_D02443 |
| Description |
Tiotropium is a long-acting, antimuscarinic bronchodilator used in the management of chronic obstructive pulmonary disease (COPD) and asthma.[A180163,L7084,L7087,L7090,L7093] Tiotropium acts mainly on M3 muscarinic receptors located in the airways to produce smooth muscle relaxation and bronchodilation.[A180163,L7084,L7087,L7090,L7093]
Tiotropium is more specific for the subset of muscarinic receptors commonly found in the lungs than [ipratropium].[A180163]
Tiotropium was granted FDA approval on 30 January 2004.[L7084] |
| Indications and Usage |
Tiotropium powder for inhalation is indicated for the maintenance of bronchospasm in COPD and to prevent exacerbations of COPD.[L7084] A combination tiotropium and [olodaterol] metered inhalation spray is indicated for maintenance of COPD.[L7087] A tiotropium inhalation spray is indicated for the maintenance of bronchospasm in COPD, to prevent exacerbations of COPD, and to treat asthma in patients 12 or more years old.[L7090] A tiotropium metered inhalation spray is indicated for the maintenance of bronchospasm in COPD, to prevent exacerbations of COPD, and to treat asthma in patients 6 or more years old.[L7093] |
| Marketing Status |
approved |
| ATC Code |
R03BB04 |
| DrugBank ID |
DB01409
|
| KEGG ID |
D01929
|
| MeSH ID |
D000069447
|
| PubChem ID |
5487427
|
| TTD Drug ID |
D0P1WA
|
| NDC Product Code |
Not Available |
| UNII |
0EB439235F
|
| Synonyms |
Tiotropium Bromide | Bromide, Tiotropium | 7-((hydroxybis(2-thienyl)acetyl)oxy)-9,9-dimethyl-3-oxa-9-azoniatricyclo(3.3.1.0(2,4))nonane bromide | Tiotropium | Spiriva | BA 679 BR | 679 BR, BA | BR, BA 679 | BA-679 BR | BA679 BR |
|
| Chemical Information |
| Molecular Formula |
C19H22NO4S2+ |
| CAS Registry Number |
186691-13-4 |
| SMILES |
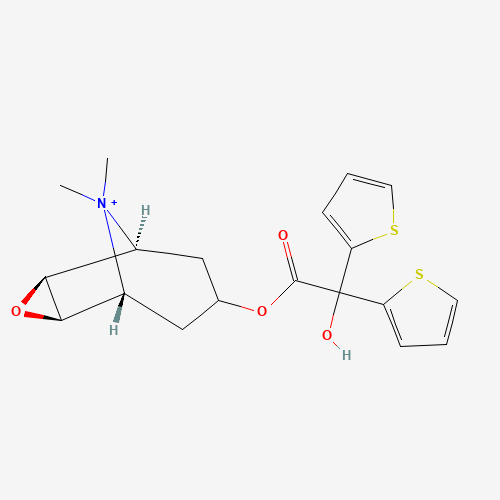
C[N+]1(C2CC(CC1C3C2O3)OC(=O)C(C4=CC=CS4)(C5=CC=CS5)O)C |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
|
|
|

