| Pharmaceutical Information |
| Drug Name |
Methylergometrine |
| Drug ID |
BADD_D02417 |
| Description |
A homolog of ergonovine containing one more CH2 group. (Merck Index, 11th ed) |
| Indications and Usage |
For the prevention and control of excessive bleeding following vaginal childbirth |
| Marketing Status |
Not Available |
| ATC Code |
G02AB01 |
| DrugBank ID |
DB00353
|
| KEGG ID |
D08207
|
| MeSH ID |
D008755
|
| PubChem ID |
8226
|
| TTD Drug ID |
D05AHE
|
| NDC Product Code |
Not Available |
| Synonyms |
Methylergonovine | Methylergometrine | Methylergobasin | Methylergometrin | Methylergonovine Maleate | Methylergometrine Maleate | Methergine | Méthergin | Methergin |
|
| Chemical Information |
| Molecular Formula |
C20H25N3O2 |
| CAS Registry Number |
113-42-8 |
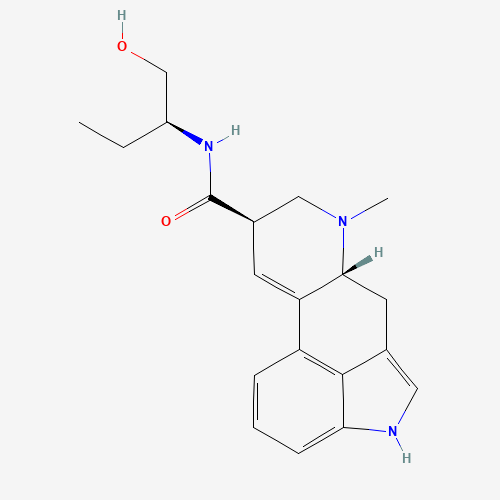
| SMILES |
CCC(CO)NC(=O)C1CN(C2CC3=CNC4=CC=CC(=C34)C2=C1)C |
| Chemical Structure |
|
|
| ADR Related Proteins Induced by Drug |
| ADR Term |
Protein Name |
UniProt AC |
TTD Target ID |
PMID |
| Not Available | Not Available | Not Available | Not Available | Not Available |
|
| ADRs Induced by Drug |
|
|

