| Pharmaceutical Information |
| Drug Name |
Ferrous sulfate |
| Drug ID |
BADD_D02403 |
| Description |
Not Available |
| Indications and Usage |
Not Available |
| Marketing Status |
Not Available |
| ATC Code |
B03AA07; B03AD03 |
| DrugBank ID |
Not Available
|
| KEGG ID |
D01725
|
| MeSH ID |
C020748
|
| PubChem ID |
24393
|
| TTD Drug ID |
Not Available
|
| NDC Product Code |
54738-963; 0220-2111 |
| Synonyms |
ferrous sulfate | ferrous sulphate | Hemobion | Plastufer | Slow-Fe | Hemofer | Vitaferro Kapseln | Aktiferrin | Apo-Ferrous Sulfate | biofer | Ceferro | Conferon | Eisendragees-ratiopharm | Eisensulfat Stada | Feospan | Fer-Gen-Sol | Fer-in-Sol | Feratab | Ferodan | Ferro-Gradumet | Kendural | Ferrograd | Ferogradumet | Fero-Gradumet | Ferrogamma | FERROinfant | ferrous sulfate, heptahydrate | iron(II) sulfate heptahydrate | Haemoprotect | Hämatopan | Mol-Iron |
|
| Chemical Information |
| Molecular Formula |
FeO4S |
| CAS Registry Number |
7720-78-7 |
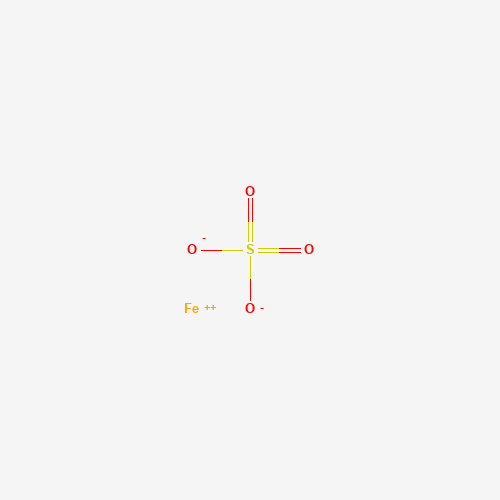
| SMILES |
[O-]S(=O)(=O)[O-].[Fe+2] |
| Chemical Structure |
|
|
| ADR Related Proteins Induced by Drug |
| ADR Term |
Protein Name |
UniProt AC |
TTD Target ID |
PMID |
| Not Available | Not Available | Not Available | Not Available | Not Available |
|
| ADRs Induced by Drug |
|
|

