| Pharmaceutical Information |
| Drug Name |
Zonisamide |
| Drug ID |
BADD_D02396 |
| Description |
Zonisamide is a sulfonamide anticonvulsant approved for use as an adjunctive therapy in adults with partial-onset seizures. Zonisamide may be a carbonic anhydrase inhibitor although this is not one of the primary mechanisms of action. Zonisamide may act by blocking repetitive firing of voltage-gated sodium channels leading to a reduction of T-type calcium channel currents, or by binding allosterically to GABA receptors. This latter action may inhibit the uptake of the inhibitory neurotransmitter GABA while enhancing the uptake of the excitatory neurotransmitter glutamate. |
| Indications and Usage |
For use as adjunctive treatment of partial seizures in adults with epilepsy. |
| Marketing Status |
approved; investigational |
| ATC Code |
N03AX15 |
| DrugBank ID |
DB00909
|
| KEGG ID |
D00538
|
| MeSH ID |
D000078305
|
| PubChem ID |
5734
|
| TTD Drug ID |
D09ZIS
|
| NDC Product Code |
76282-228; 13672-001; 60510-611; 65841-125; 70600-003; 59651-380; 63629-3293; 68001-242; 68462-129; 70771-1143; 72578-040; 76282-226; 38217-0040; 59651-378; 35356-143; 59212-681; 63187-897; 70518-3224; 70771-1144; 49452-9000; 76072-1012; 0615-8266; 72578-041; 60687-230; 61919-917; 62756-259; 62756-260; 17511-137; 68554-0009; 29300-430; 63187-583; 69097-861; 71335-0486; 71052-065; 52652-8001; 68001-244; 76282-227; 13672-002; 51927-0109; 51927-4807; 59361-002; 59212-680; 59651-379; 46438-0063; 60510-610; 66039-122; 73309-257; 29300-429; 62756-258; 70771-1142; 51552-1572; 73377-112; 68001-243; 68788-7438; 72578-042; 62756-334; 29300-428; 50268-816; 68462-128; 68462-130; 70518-3180; 71205-727; 71335-0962; 71335-9679; 72189-374; 38779-3045; 66039-929 |
| UNII |
459384H98V
|
| Synonyms |
Zonisamide | 3-Sulfamoylmethyl-1,2-benzisoxazole | 3 Sulfamoylmethyl 1,2 benzisoxazole | AD 810 | AD-810 | AD810 | CI 912 | CI-912 | CI912 | Zonegran | Zonisamide Monosodium |
|
| Chemical Information |
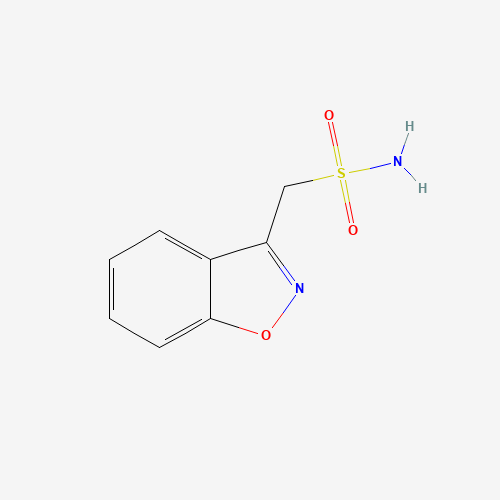
| Molecular Formula |
C8H8N2O3S |
| CAS Registry Number |
68291-97-4 |
| SMILES |
C1=CC=C2C(=C1)C(=NO2)CS(=O)(=O)N |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Renal vein varices | 24.10.04.006; 20.01.07.023 | 0.000414% | | Not Available | | SJS-TEN overlap | 23.03.01.041; 12.03.01.069; 11.07.01.030; 10.01.01.043 | 0.000414% | | Not Available |
|
|
|

