| Pharmaceutical Information |
| Drug Name |
Ziconotide |
| Drug ID |
BADD_D02381 |
| Description |
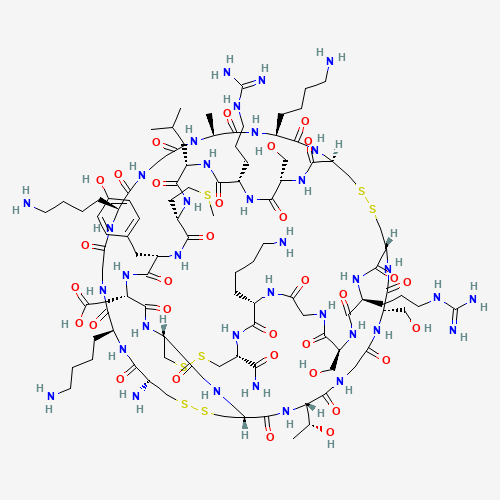
Ziconotide (also known as SNX-111) is a neurotoxic peptide derived from the cone snail _Conus magus_ comprising 25 amino acids with three disulphide bonds.[A202835, L13389] Other such peptides, collectively termed conotoxins, exist, and some have shown efficacy in binding specific subsets of calcium channels; ziconotide is used in part because it can be synthesized without loss of proper bond formation or structural elements.[A202829, A202832] Ziconotide is used to manage severe chronic pain refractory to other methods, through its ability to inhibit N-type calcium channels involved in nociceptive signalling.[A202829, A202835, A202838, A202841, A202850, A202859, L13389]
Ziconotide was granted FDA approval on December 28, 2004 for marketing by TerSera therapeutics LLC. under the name Prialt.[L13389] To date, ziconotide is the only calcium channel blocking peptide approved for use by the FDA.[A202835] |
| Indications and Usage |
Ziconotide is indicated for the management of severe chronic pain in patients refractory to other treatments, and for whom intrathecal therapy is warranted.[L13389] |
| Marketing Status |
approved |
| ATC Code |
N02BG08 |
| DrugBank ID |
DB06283
|
| KEGG ID |
D06363
|
| MeSH ID |
C078452
|
| PubChem ID |
16135415
|
| TTD Drug ID |
D01NLB
|
| NDC Product Code |
52416-777 |
| UNII |
7I64C51O16
|
| Synonyms |
ziconotide | CYS-LYS-GLY-LYS-GLY-ALA-LYS-CYS-SER-ARG-LEU-MET-TYR-ASP-CYS-CYS-THR-GLY-SER-CYS-ARG-SER-GLY-LYS-CYS-NH2 | omega-conotoxin MVIIA | omega-conotoxin M VIIA | omega-conopeptide MVIIA | leconotide | SNX 111 | SNX-111 | omega-conotoxin MVIIA, Conus magus | Prialt |
|
| Chemical Information |
| Molecular Formula |
C102H172N36O32S7 |
| CAS Registry Number |
107452-89-1 |
| SMILES |
CC1C(=O)NC(C(=O)NC2CSSCC3C(=O)NC(C(=O)NC(C(=O)NCC(=O)NC(C(=O)NC(CSSCC(C(=O)NC(CS
SCC(C(=O)NC(C(=O)NCC(=O)NC(C(=O)NCC(=O)N1)CCCCN)CCCCN)N)C(=O)NC(C(=O)NCC(=O)NC(C
(=O)N3)CO)C(C)O)NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC2=O)CO)CCCNC(=
N)N)CC(C)C)CCSC)CC4=CC=C(C=C4)O)CC(=O)O)C(=O)N)CCCCN)CO)CCCNC(=N)N)CCCCN |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

