| Pharmaceutical Information |
| Drug Name |
Water |
| Drug ID |
BADD_D02375 |
| Description |
Water (chemical formula: H2O) is a transparent fluid which forms the world's streams, lakes, oceans and rain, and is the major constituent of the fluids of organisms. As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. Water is a liquid at standard ambient temperature and pressure, but it often co-exists on Earth with its solid state, ice; and gaseous state, steam (water vapor). |
| Indications and Usage |
For diluting or dissolving drugs for intravenous, intramuscular or subcutaneous injection, according to instructions of the manufacturer of the drug to be administered [FDA Label]. |
| Marketing Status |
approved |
| ATC Code |
Not Available |
| DrugBank ID |
DB09145
|
| KEGG ID |
D00001
|
| MeSH ID |
D014867
|
| PubChem ID |
962
|
| TTD Drug ID |
Not Available
|
| NDC Product Code |
72745-101; 52919-033; 81483-9031; 13985-609; 0173-0857; 72572-747; 0990-7118; 52919-014; 55718-120; 43598-126; 52584-977; 57687-226; 57687-276; 0338-0013; 0404-9971; 0924-0165; 0990-7139; 17271-750; 47682-410; 58264-0272; 0404-7198; 0404-9970; 59779-337; 65785-162; 76420-082; 0990-6139; 0990-7973; 52919-003; 76297-002; 0264-7850; 55154-0127; 63323-178; 69183-230; 0498-0601; 71631-0004; 71872-7182; 52919-009; 52919-032; 69227-205; 49873-020; 50242-001; 52584-887; 0264-2101; 57349-220; 63868-841; 65785-164; 66302-150; 0409-4887; 0517-3010; 52919-034; 55718-121; 47682-412; 0264-7385; 64108-313; 65785-166; 67691-770; 0517-3020; 71872-7041; 71872-7129; 52919-008; 63552-134; 11822-9860; 52584-079; 0338-0003; 63323-185; 65219-187; 65785-160; 72745-100; 0641-6147; 82001-001; 52919-005; 55718-122; 42961-300; 0338-0004; 71631-0003; 17478-620; 47682-936; 49687-0010; 65785-169; 0404-9959; 0990-7990; 52919-006; 52919-011 |
| UNII |
059QF0KO0R
|
| Synonyms |
Water | Hydrogen Oxide |
|
| Chemical Information |
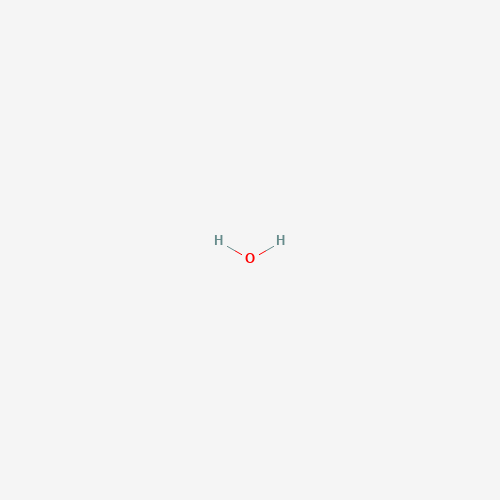
| Molecular Formula |
H2O |
| CAS Registry Number |
7732-18-5 |
| SMILES |
O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

