| Pharmaceutical Information |
| Drug Name |
Voriconazole |
| Drug ID |
BADD_D02369 |
| Description |
Voriconazole (Vfend, Pfizer) is a triazole antifungal medication used to treat serious fungal infections.[L15571] It is used to treat invasive fungal infections that are generally seen in patients who are immunocompromised. These include invasive candidiasis, invasive aspergillosis, and emerging fungal infections. The increased affinity of voriconazole for 14-alpha sterol demethylase makes it useful against some [fluconazole]-resistant organisms.[A236050]
Voriconazole was approved by the FDA under the trade name Vfend on May 24, 2002.[L34660] |
| Indications and Usage |
For the treatment of esophageal candidiasis, cadidemia, invasive pulmonary aspergillosis, and serious fungal infections caused by Scedosporium apiospermum and Fusarium spp.[L9821, L15571] |
| Marketing Status |
approved |
| ATC Code |
J02AC03 |
| DrugBank ID |
DB00582
|
| KEGG ID |
D00578
|
| MeSH ID |
D065819
|
| PubChem ID |
71616
|
| TTD Drug ID |
D0N3VR
|
| NDC Product Code |
0781-5667; 60715-1700; 65372-1175; 27241-063; 39822-1077; 43386-088; 0378-1640; 66039-951; 70966-0030; 47781-466; 0049-4190; 55111-099; 58032-2007; 66039-876; 43386-038; 51079-164; 0049-3190; 64980-274; 64980-275; 65862-891; 68462-572; 70594-067; 70710-1247; 60687-273; 0378-1626; 65841-830; 72572-875; 68554-0111; 75945-102; 16714-199; 43547-378; 59762-0936; 63739-008; 65162-913; 65841-831; 0904-6596; 65862-916; 71052-149; 40032-038; 43386-089; 50268-803; 0049-3180; 68083-321; 71288-027; 72578-062; 14501-0014; 52562-001; 27241-062; 43547-377; 51552-1524; 16714-198; 59762-0934; 60687-294; 65219-190; 65862-892; 70771-1413; 72843-441; 0781-5668; 63126-911; 0049-3170; 70436-029; 72578-063; 0781-3416; 0904-7024; 51552-1573; 53296-0081; 64220-140; 65015-801; 73223-005; 51079-165; 63629-8734; 0049-3160; 68462-573; 72266-131; 51407-133; 55150-321; 59762-0935 |
| UNII |
JFU09I87TR
|
| Synonyms |
Voriconazole | UK 109,496 | UK-109,496 | UK109,496 | UK-109496 | UK109496 | UK 109496 | Vfend |
|
| Chemical Information |
| Molecular Formula |
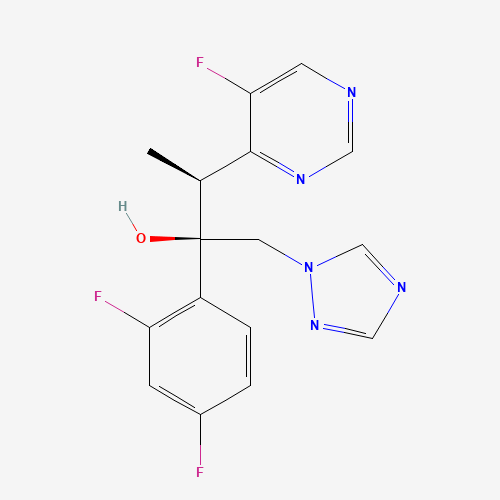
C16H14F3N5O |
| CAS Registry Number |
188416-29-7 |
| SMILES |
CC(C1=NC=NC=C1F)C(CN2C=NC=N2)(C3=C(C=C(C=C3)F)F)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

