| Pharmaceutical Information |
| Drug Name |
Urea |
| Drug ID |
BADD_D02318 |
| Description |
A compound formed in the liver from ammonia produced by the deamination of amino acids. It is the principal end product of protein catabolism and constitutes about one half of the total urinary solids. |
| Indications and Usage |
- 10% hydrate skin
- 15% accelerate fibrin degradation
- 20-30% are antipruritic, break down keratin, decrease the thickness of the stratum corneum and are used in scaling conditions such as ichthysosis
- 40% are proteolytic and may be used to dissolve and peel dystrophic nails
[Patient Self Care, 2010] |
| Marketing Status |
approved; investigational |
| ATC Code |
B05BC02; D02AE01 |
| DrugBank ID |
DB03904
|
| KEGG ID |
D00023; D01749
|
| MeSH ID |
D014508
|
| PubChem ID |
1176
|
| TTD Drug ID |
D02XBW
|
| NDC Product Code |
64173-202; 42808-204; 59088-489; 14639-8432; 50268-872; 61344-455; 63629-2038; 42792-101; 59088-698; 63629-9266; 58657-487; 63629-9267; 50268-820; 63629-2036; 69437-341; 54295-312; 63629-9265; 71399-8455; 16477-340; 42192-115; 42808-202; 42291-849; 44523-617; 54295-308; 63629-2040; 81542-201; 0904-7167; 16477-341; 71399-8456; 82429-308; 49452-8073; 51552-0057; 50096-501; 54295-311; 61344-452; 68134-801; 51552-1522; 52187-549 |
| UNII |
8W8T17847W
|
| Synonyms |
Urea | Carbamide | Basodexan | Carmol |
|
| Chemical Information |
| Molecular Formula |
CH4N2O |
| CAS Registry Number |
57-13-6 |
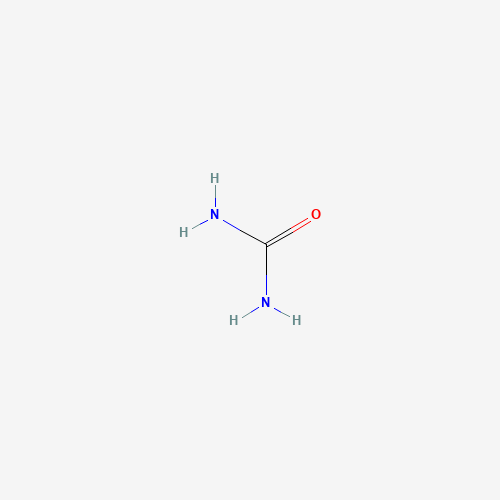
| SMILES |
C(=O)(N)N |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Burning sensation | 17.02.06.001; 08.01.09.029 | - | - | Not Available | | Irritability | 08.01.03.011; 19.04.02.013 | - | - | | | Pain | 08.01.08.004 | - | - | | | Pruritus | 23.03.12.001 | - | - | | | Skin irritation | 23.03.04.009 | - | - | Not Available |
|
The 1th Page
1
Total 1 Pages
|
|

