| Pharmaceutical Information |
| Drug Name |
Teduglutide recombinant |
| Drug ID |
BADD_D02138 |
| Description |
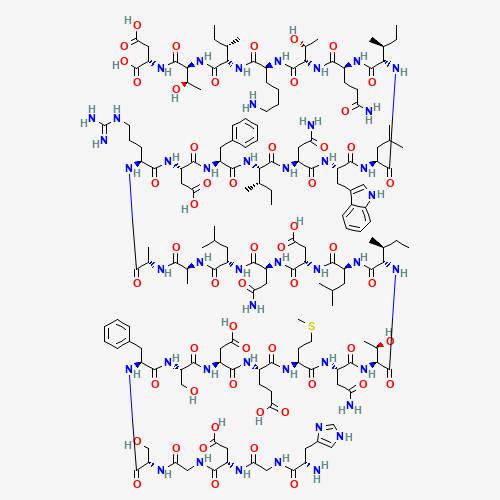
Teduglutide is a glucagon-like peptide-2 (GLP-2) analogue. It is made up of 33 amino acids and is manufactured using a strain of Escherichia coli modified by recombinant DNA technology. Teduglutide differs from GLP-2 by one amino acid (alanine is substituted by glycine). The significance of this substitution is that teduglutide is longer acting than endogenous GLP-2 as it is more resistant to proteolysis from dipeptidyl peptidase-4. FDA approved on December 21, 2012. |
| Indications and Usage |
Treatment of short bowel syndrome (SBS), malabsorption associated with the removal of the intestine, in adults patients who are dependent on parenteral support. |
| Marketing Status |
approved |
| ATC Code |
A16AX08 |
| DrugBank ID |
DB08900
|
| KEGG ID |
D06053
|
| MeSH ID |
C494910
|
| PubChem ID |
70683012
|
| TTD Drug ID |
D00RCI
|
| NDC Product Code |
63557-1031; 68225-087; 68875-0101; 68875-0102; 69766-106; 68594-001; 41524-0009; 68875-0103; 17337-0264 |
| UNII |
Not Available
|
| Synonyms |
teduglutide | Gly(2)-GLP-2 | (Gly2)GLP-2 |
|
| Chemical Information |
| Molecular Formula |
C164H252N44O55S |
| CAS Registry Number |
197922-42-2 |
| SMILES |
CCC(C)C(C(=O)NC(C(C)O)C(=O)NC(CC(=O)O)C(=O)O)NC(=O)C(CCCCN)NC(=O)C(C(C)O)NC(=O)C
(CCC(=O)N)NC(=O)C(C(C)CC)NC(=O)C(CC(C)C)NC(=O)C(CC1=CNC2=CC=CC=C21)NC(=O)C(CC(=O
)N)NC(=O)C(C(C)CC)NC(=O)C(CC3=CC=CC=C3)NC(=O)C(CC(=O)O)NC(=O)C(CCCNC(=N)N)NC(=O)
C(C)NC(=O)C(C)NC(=O)C(CC(C)C)NC(=O)C(CC(=O)N)NC(=O)C(CC(=O)O)NC(=O)C(CC(C)C)NC(=
O)C(C(C)CC)NC(=O)C(C(C)O)NC(=O)C(CC(=O)N)NC(=O)C(CCSC)NC(=O)C(CCC(=O)O)NC(=O)C(C
C(=O)O)NC(=O)C(CO)NC(=O)C(CC4=CC=CC=C4)NC(=O)C(CO)NC(=O)CNC(=O)C(CC(=O)O)NC(=O)C
NC(=O)C(CC5=CNC=N5)N |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

