| Pharmaceutical Information |
| Drug Name |
Spironolactone |
| Drug ID |
BADD_D02066 |
| Description |
Spironolactone is a potassium sparing diuretic like [eplerenone] that competitively inhibits mineralocorticoid receptors in the distal convoluted tubule to promote sodium and water excretion and potassium retention.[A11837]. Spironolactone was originally developed purely for this ability before other pharmacodynamic properties of the drug were discovered.[A11837,A178246] It is indicated to treat a number of conditions including heart failure, deem, hyperaldosteronism, adrenal hyperplasia, hypertension, and nephrotic syndrome.[Label] Off label uses of spironolactone involving its antiandrogenic activity include hirsutism, female pattern hair loss, and adult acne vulgaris.[A178135] Spironolactone is also frequently used in medical gender transition.[A178138]
Spironolactone was developed in 1957, marketed in 1959, and approved by the FDA on January 21, 1960.[A178243,L6187] |
| Indications and Usage |
Spironolactone is indicated for the treatment of New York Heart Association Class III-IV heart failure, management of edema in cirrhotic adults not responsive to fluid and sodium restrictions, primary hyperaldosteronism short-term preoperatively, primary hyperaldosteronism long-term in patients with aldosterone producing adrenal adenomas that are not candidates for surgery or patients with bilarteral micro/macronodular adrenal hyperplasia, as an add-on therapy in hypertension, and in nephrotic syndrome when treatment of the disease as well as fluid and sodium restriction with other diuretics is inadequate.[Label]
Spironolactone has antiandrogenic activity which leads to many of its off label uses. Spironolactone is used off label in the treatment of hirsutism, female pattern hair loss, and adult acne vulgaris.[A178135]
Spironolactone is also frequently used for its antiandrogenic effects in transgender female patients due to its low cost and reducing male-pattern hair growth.[A178138] |
| Marketing Status |
approved |
| ATC Code |
C03DA01 |
| DrugBank ID |
DB00421
|
| KEGG ID |
D00443
|
| MeSH ID |
D013148
|
| PubChem ID |
5833
|
| TTD Drug ID |
D0EP0C
|
| NDC Product Code |
72603-136; 72789-292; 43063-832; 50090-6458; 0025-1041; 59746-216; 63629-1093; 63629-1830; 67296-1403; 68071-2977; 69584-853; 70518-0563; 70518-2585; 70771-1029; 71205-147; 71205-772; 76420-556; 76420-558; 79572-032; 16714-639; 43063-974; 50090-3580; 51655-153; 53489-329; 53746-515; 59651-428; 59746-218; 63629-1092; 63629-1094; 63629-2437; 0378-2146; 68071-2606; 68382-661; 70518-1334; 70518-2386; 70518-2588; 71335-0401; 72189-497; 0904-6927; 42708-126; 51655-861; 53746-514; 55700-953; 0025-1031; 60687-476; 63629-1065; 63629-1066; 63629-2438; 63629-8539; 65162-515; 68071-2968; 68071-2989; 68788-7297; 69584-852; 70518-1746; 71610-288; 55154-5517; 59746-217; 60687-465; 63187-841; 67544-310; 68382-660; 69584-854; 70518-3118; 70518-3625; 70518-3750; 0615-8178; 0615-8221; 0615-8451; 38779-0096; 57582-030; 82298-121; 50090-3747; 50090-6432; 50090-6539; 53002-4720; 55700-968; 0025-1001; 63629-2436; 68071-2936; 68071-2962; 68071-2988; 68788-7019; 68788-8381; 68788-8452; 70518-0603; 70518-2461; 70518-3655; 71205-146; 71335-0966; 71610-287; 72162-1626; 76420-557; 16714-637; 16729-225; 51655-344; 51655-636; 53002-1707; 54348-340; 63187-634; 63187-861; 63629-1067; 63739-545; 67296-1692; 70518-0455; 71335-0053; 51552-0276; 51927-1377; 55525-0002; 61907-031; 70966-0028; 16714-085; 16714-086; 59651-426; 63629-1064; 63629-1095; 63629-4094; 71205-148; 76420-063; 49452-7220; 16729-227; 51079-103; 55154-3556; 60687-487; 61919-772; 63629-1061; 63629-1062; 68382-662; 68788-7051; 70518-2384; 70518-2385; 72162-1624; 72603-134; 72789-291; 50090-1292; 50090-6408; 53746-511; 59651-427; 0378-0437; 65162-511; 65162-514; 70518-1314; 70771-1027; 70771-1028; 71335-0304; 72162-1627; 72189-495; 0615-8452; 72789-290; 16714-084; 16729-226; 42708-101; 50090-0136; 50090-6369; 51079-979; 53489-328; 55154-1495; 63629-5341; 63739-544; 0378-0243; 70518-1742; 70518-2589; 70518-3721; 71610-102; 72189-329; 72189-496; 72603-135; 76420-062; 16637-0020; 45541-1134; 16714-638; 43063-835; 50090-1308; 53489-143 |
| UNII |
27O7W4T232
|
| Synonyms |
Spironolactone | Spirolactone | Veroshpiron | Verospirone | Spiractin | Spirobeta | Spirogamma | Spirolang | Spirono-Isis | Spirono Isis | Spironone | Spirospare | Aldactone | Verospiron | Aldactone A | Aquareduct | Duraspiron | Espironolactona Alter | Espironolactona Mundogen | Flumach | Frumikal | Jenaspiron | Novo-Spiroton | Novo Spiroton | NovoSpiroton | Practon | SC-9420 | SC 9420 | SC9420 | Spiro L.U.T. | Spiro Von Ct | Ct, Spiro Von | Von Ct, Spiro |
|
| Chemical Information |
| Molecular Formula |
C24H32O4S |
| CAS Registry Number |
52-01-7 |
| SMILES |
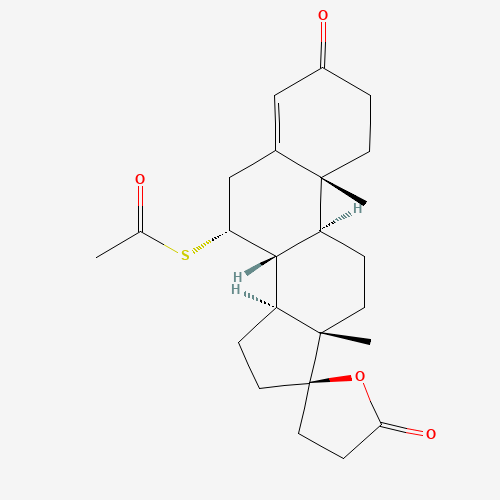
CC(=O)SC1CC2=CC(=O)CCC2(C3C1C4CCC5(C4(CC3)C)CCC(=O)O5)C |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
|
|
|

