| Pharmaceutical Information |
| Drug Name |
Somatuline depot |
| Drug ID |
BADD_D02056 |
| Description |
Lanreotide is a drug employed in the management of acromegaly (a hormonal condition caused by excess growth hormone) in addition to symptoms caused by neuroendocrine tumors, especially carcinoid syndrome. This drug is a long-acting analog of the drug somatostatin, a growth hormone inhibitor. Lanreotide is manufactured by the company, _Ipsen Pharmaceuticals_ as lanreotide acetate, and marketed as _Somatuline_. It is approved in several countries worldwide, including the United Kingdom, Australia, and Canada. Lanreotide was first approved for use in the United States by the FDA on August 30, 2007. |
| Indications and Usage |
Lanreotide is a somatostatin analog approved for treatment of neuroendocrine tumours and acromegaly. |
| Marketing Status |
approved |
| ATC Code |
H01CB03 |
| DrugBank ID |
DB06791
|
| KEGG ID |
D04666
|
| MeSH ID |
C060347
|
| PubChem ID |
6918010
|
| TTD Drug ID |
D0M2YE
|
| NDC Product Code |
52416-117 |
| UNII |
Not Available
|
| Synonyms |
lanreotide | 3-(2-naphthyl)alanyl-cystinyl-tyrosyl-tryptophyl-lysyl-valyl-cystinyl-threonine amide | D-Nal-Cys-Tyr-Trp-Lys-Val-Cys-Thr-NH2 | angiopeptin | naphthalenyl-cyclo(cysteinyl-tyrosyl-tryptophyl-lysyl-valyl-cysteinyl)threoninamide | L-Threoninamide, 3-(2-naphthalenyl)-D-alanyl-L-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-valyl-L-cysteinyl-, cyclic (2-7)-disulfide | Nal-cyclo(Cys-Tyr-Trp-Lys-Val-Cys)-Thr-NH2 | Nal-cyclo(Cys-Tyr-Trp-Lys-Val-Cys)Thr-NH2 | 2-naphthylalanyl-cyclo(cysteinyl-tyrosyl-tryptophyl-lysyl-valyl-cysteinyl)-threoninamide | 3-(2-naphthyl)-D-Ala-Cys-Tyr-D-Trp-Lys-Val-Cys-Thr-NH2 | naphthyl-cyclo(Cys-Tyr-Trp-Lys-Val-Cys)Thr-NH2 | BIM 23014 | BIM-23014 | DC 13-116 | DC13-116 | DC-13-116 | lanreotide acetate | Somatulina | Somatuline | lanreotide-SR | BIM 23014 C | BIM 23014C | BIM-23014 C | 188Re-lanreotide | Somatulin |
|
| Chemical Information |
| Molecular Formula |
C56H73N11O12S2 |
| CAS Registry Number |
127984-74-1 |
| SMILES |
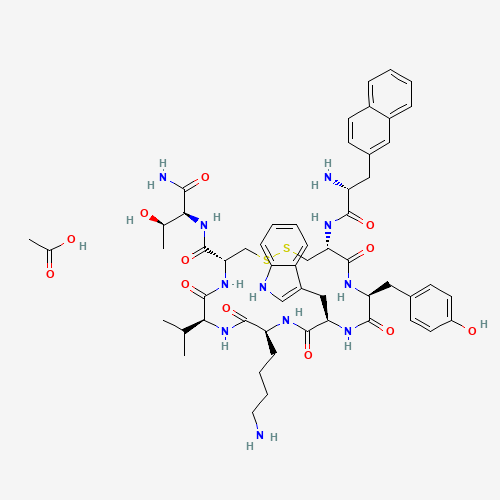
CC(C)C1C(=O)NC(CSSCC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N1)CCCCN)CC2=CNC3=CC=CC=C32)CC
4=CC=C(C=C4)O)NC(=O)C(CC5=CC6=CC=CC=C6C=C5)N)C(=O)NC(C(C)O)C(=O)N.CC(=O)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

