| Pharmaceutical Information |
| Drug Name |
Sibutramine |
| Drug ID |
BADD_D02016 |
| Description |
Sibutramine (trade name Meridia in the USA, Reductil in Europe and other countries), usually as sibutramide hydrochloride monohydrate, is an orally administered agent for the treatment of obesity. It is a centrally acting stimulant chemically related to amphetamines thus it is classified as a Schedule IV controlled substance in the United States. In October 2010, Sibutramine was withdrawn from Canadian and U.S. markets due to concerns that the drug increases the risk of heart attack and stroke in patients with a history of heart disease. |
| Indications and Usage |
For the treatment of obesity. |
| Marketing Status |
approved; illicit; investigational; withdrawn |
| ATC Code |
A08AA10 |
| DrugBank ID |
DB01105
|
| KEGG ID |
D08513
|
| MeSH ID |
C058254
|
| PubChem ID |
5210
|
| TTD Drug ID |
D08KVZ
|
| NDC Product Code |
Not Available |
| UNII |
WV5EC51866
|
| Synonyms |
sibutramine | di-desmethylsibutramine | didesmethylsibutramine | (R)-DDMS | Reductil | mono-desmethylsibutramine | sibutramine hydrochloride | N-1-(1-(4-chlorophenyl)cyclobutyl)-3-methylbutyl-N,N-dimethylamine HCl | BTS 54 524 | BTS-54524 | BTS 54524 | Meridia |
|
| Chemical Information |
| Molecular Formula |
C17H26ClN |
| CAS Registry Number |
106650-56-0 |
| SMILES |
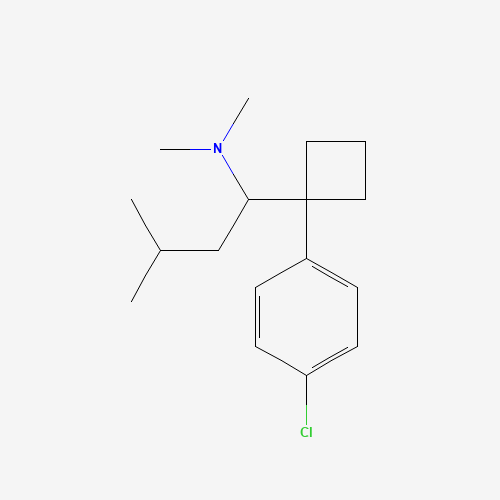
CC(C)CC(C1(CCC1)C2=CC=C(C=C2)Cl)N(C)C |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Intermenstrual bleeding | 21.01.01.015 | - | - | Not Available |
|
|
|

