| Pharmaceutical Information |
| Drug Name |
Sertraline |
| Drug ID |
BADD_D02010 |
| Description |
Sertraline is a popular antidepressant medication commonly known as a selective serotonin reuptake inhibitor (SSRI), and is similar to drugs such as [Citalopram] and [Fluoxetine]. Despite marked structural differences between compounds in this drug class, SSRIs exert similar pharmacological effects.
Several weeks of therapy with sertraline may be required before beneficial effects are noticed. Sertraline displays enhanced safety or tolerability than other classes of antidepressants, which frequently cause high levels of drowsiness, dizziness, blurred vision, and other undesirable effects.[A1846,A187075,T28] |
| Indications and Usage |
Sertraline is indicated for the management of major depressive disorder (MDD), post-traumatic stress disorder (PTSD), obsessive-compulsive disorder (OCD), panic disorder (PD), premenstrual dysphoric disorder (PMDD), and social anxiety disorder (SAD).[L9016] Common off-label uses for sertraline include the prevention of post stroke depression[A187078], generalized anxiety disorder (GAD), fibromyalgia, premature ejaculation, migraine prophylaxis, diabetic neuropathy, and neurocardiogenic syncope.[L5227]
|
| Marketing Status |
approved |
| ATC Code |
N06AB06 |
| DrugBank ID |
DB01104
|
| KEGG ID |
D02360
|
| MeSH ID |
D020280
|
| PubChem ID |
68617
|
| TTD Drug ID |
D0K0TC
|
| NDC Product Code |
76282-214; 23155-759; 68180-353; 55700-225; 63187-055; 70518-0153; 70518-1461; 70518-1617; 71335-9714; 0615-7990; 65427-009; 55154-3570; 0615-7991; 76282-212; 0904-6924; 50090-5848; 70518-1040; 23155-757; 70518-2512; 0904-6925; 68180-351; 68180-352; 68071-4400; 63187-212; 68788-7329; 0615-7989; 0904-6926; 55154-3569; 63187-478; 67296-1199; 70934-958; 76282-213; 23155-758; 50090-0989 |
| UNII |
QUC7NX6WMB
|
| Synonyms |
Sertraline | Zoloft | Altruline | Lustral | Apo-Sertraline | Apo Sertraline | Aremis | Besitran | Sealdin | Gladem | Novo-Sertraline | Novo Sertraline | ratio-Sertraline | ratio Sertraline | Rhoxal-sertraline | Rhoxal sertraline | Sertraline Hydrochloride | Hydrochloride, Sertraline | Sertraline Hydrochloride (1S-cis)-Isomer | Gen-Sertraline | Gen Sertraline |
|
| Chemical Information |
| Molecular Formula |
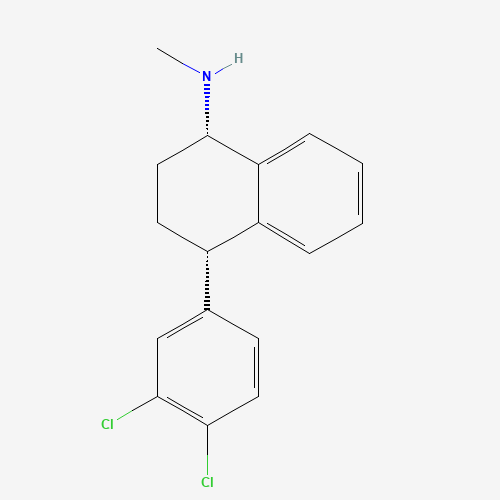
C17H17Cl2N |
| CAS Registry Number |
79617-96-2 |
| SMILES |
CNC1CCC(C2=CC=CC=C12)C3=CC(=C(C=C3)Cl)Cl |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
|
|
|

